Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide
- PMID: 12591899
- PMCID: PMC2193858
- DOI: 10.1084/jem.20021633
Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide
Abstract
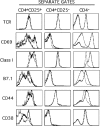
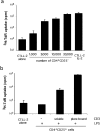
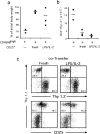
Regulatory CD4 T cells (Treg) control inflammatory reactions to commensal bacteria and opportunist pathogens. Activation of Treg functions during these processes might be mediated by host-derived proinflammatory molecules or directly by bacterial products. We tested the hypothesis that engagement of germline-encoded receptors expressed by Treg participate in the triggering of their function. We report that the subset of CD4 cells known to exert regulatory functions in vivo (CD45RB(low) CD25(+)) selectively express Toll-like receptors (TLR)-4, -5, -7, and -8. Exposure of CD4(+) CD25(+) cells to the TLR-4 ligand lipopolysaccharide (LPS) induces up-regulation of several activation markers and enhances their survival/proliferation. This proliferative response does not require antigen-presenting cells and is augmented by T cell receptor triggering and interleukin 2 stimulation. Most importantly, LPS treatment increases CD4(+) CD25(+) cell suppressor efficiency by 10-fold and reveals suppressive activity in the CD4(+) CD45RB(low) CD25(-) subset that when tested ex-vivo, scores negative. Moreover, LPS-activated Treg efficiently control naive CD4 T cell-dependent wasting disease. These findings provide the first evidence that Treg respond directly to proinflammatory bacterial products, a mechanism that likely contributes to the control of inflammatory responses.
Figures

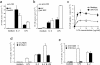

Comment in
-
Control of immune responses by naturally arising CD4+ regulatory T cells that express toll-like receptors.J Exp Med. 2003 Feb 17;197(4):397-401. doi: 10.1084/jem.20030012. J Exp Med. 2003. PMID: 12591898 Free PMC article. No abstract available.
References
-
- Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. - PubMed
-
- Hori, S., T.L. Carvalho, and J. Demengeot. 2002. CD25+ CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282–1291. - PubMed
-
- Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L.R. Brunet, D.M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625–629. - PubMed
-
- McGuirk, P., C. McCann, and K.H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221–231. - PMC - PubMed
-
- Coutinho, A., S. Hori, T. Carvalho, I. Caramalho, and J. Demengeot. 2001. Regulatory T cells: the physiology of autoreactivity in dominant tolerance and “quality control” of immune responses. Immunol. Rev. 182:89–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

