Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis
- PMID: 12591904
- PMCID: PMC2193869
- DOI: 10.1084/jem.20021477
Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis
Abstract
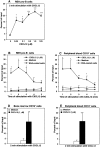
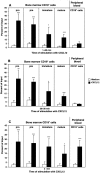
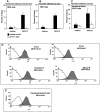
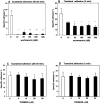
It is largely unknown how hematopoietic progenitors are positioned within specialized niches of the bone marrow microenvironment during development. Chemokines such as CXCL12, previously called stromal cell-derived factor 1, are known to activate cell integrins of circulating leukocytes resulting in transient adhesion before extravasation into tissues. However, this short-term effect does not explain the mechanism by which progenitor cells are retained for prolonged periods in the bone marrow. Here we show that in human bone marrow CXCL12 triggers a sustained adhesion response specifically in progenitor (pro- and pre-) B cells. This sustained adhesion diminishes during B cell maturation in the bone marrow and, strikingly, is absent in circulating mature B cells, which exhibit only transient CXCL12-induced adhesion. The duration of adhesion is tightly correlated with CXCL12-induced activation of focal adhesion kinase (FAK), a known molecule involved in integrin-mediated signaling. Sustained adhesion of progenitor B cells is associated with prolonged FAK activation, whereas transient adhesion in circulating B cells is associated with short-lived FAK activation. Moreover, sustained and transient adhesion responses are differentially affected by pharmacological inhibitors of protein kinase C and phosphatidylinositol 3-kinase. These results provide a developmental cell stage-specific mechanism by which chemokines orchestrate hematopoiesis through sustained rather than transient activation of adhesion and cell survival pathways.
Figures




Similar articles
-
CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in pro-B cells.J Immunol. 2005 Mar 1;174(5):2582-90. doi: 10.4049/jimmunol.174.5.2582. J Immunol. 2005. PMID: 15728464
-
Focal adhesion kinase is required for CXCL12-induced chemotactic and pro-adhesive responses in hematopoietic precursor cells.Leukemia. 2007 Aug;21(8):1723-32. doi: 10.1038/sj.leu.2404769. Epub 2007 Jun 14. Leukemia. 2007. PMID: 17568820
-
Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C.Blood. 2000 Apr 15;95(8):2505-13. Blood. 2000. PMID: 10753828
-
Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow.J Leukoc Biol. 2001 Jun;69(6):860-6. J Leukoc Biol. 2001. PMID: 11404368 Review.
-
VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells.Leuk Lymphoma. 1997 Feb;24(5-6):423-35. doi: 10.3109/10428199709055581. Leuk Lymphoma. 1997. PMID: 9086434 Review.
Cited by
-
Transforming growth factor-{beta}1 modulates responses of CD34+ cord blood cells to stromal cell-derived factor-1/CXCL12.Blood. 2005 Jul 15;106(2):485-93. doi: 10.1182/blood-2004-10-4145. Epub 2005 Mar 29. Blood. 2005. PMID: 15797995 Free PMC article.
-
Multiple levels of chemokine receptor regulation in the control of mouse natural killer cell development.Front Immunol. 2014 Feb 13;5:44. doi: 10.3389/fimmu.2014.00044. eCollection 2014. Front Immunol. 2014. PMID: 24592263 Free PMC article. Review.
-
Toward Therapeutic Targeting of Bone Marrow Leukemic Niche Protective Signals in B-Cell Acute Lymphoblastic Leukemia.Front Oncol. 2021 Jan 8;10:606540. doi: 10.3389/fonc.2020.606540. eCollection 2020. Front Oncol. 2021. PMID: 33489914 Free PMC article. Review.
-
Murine Bone Marrow Niches from Hematopoietic Stem Cells to B Cells.Int J Mol Sci. 2018 Aug 10;19(8):2353. doi: 10.3390/ijms19082353. Int J Mol Sci. 2018. PMID: 30103411 Free PMC article. Review.
-
Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells.J Clin Invest. 2005 Jan;115(1):168-76. doi: 10.1172/JCI21773. J Clin Invest. 2005. PMID: 15630457 Free PMC article.
References
-
- Murphy, P.M. 1994. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12:593–633. - PubMed
-
- Mackay, C.R. 2001. Chemokines: immunology's high impact factors. Nat. Immunol. 2:95–101. - PubMed
-
- Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217–242. - PubMed
-
- Gunn, M.D., V.N. Ngo, K.M. Ansel, E.H. Ekland, J.G. Cyster, and L.T. Williams. 1998. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 391:799–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

