In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells
- PMID: 12591905
- PMCID: PMC2193862
- DOI: 10.1084/jem.20021765
In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells
Abstract
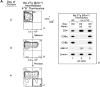
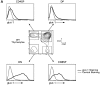
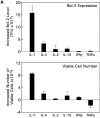
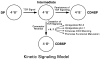
CD4(+)8(+) double positive (DP) thymocytes differentiate into CD4(+) and CD8(+) mature T cells in response to TCR signals. However, TCR signals that are initiated in DP thymocytes are unlikely to persist throughout all subsequent differentiation steps, suggesting that other signals must sustain thymocyte differentiation after TCR signaling has ceased. Using an in vitro experimental system, we now demonstrate that cytokine receptor signals, such as those transduced by IL-7 receptors, are required for differentiation of signaled DP thymocytes into functionally mature CD8(+) T cells as they: (a) up-regulate Bcl-2 expression to maintain thymocyte viability; (b) enhance CD4 gene silencing; (c) promote functional maturation;and (d) up-regulate surface expression of glucose transporter molecules, which improve nutrient uptake and increase metabolic activity. IL-7Rs appear to be unique among cytokine receptors in maintaining the viability of newly generated CD4(-)8(+) thymocytes, whereas several different cytokine receptors can provide the trophic/differentiative signals for subsequent CD8(+) thymocyte differentiation and maturation. Thus, cytokine receptors provide both survival and trophic/differentiative signals with varying degrees of redundancy that are required for differentiation of signaled DP thymocytes into functionally mature CD8(+) T cells.
Figures













References
-
- Jameson, S.C., K.A. Hogquist, and M.J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93–126. - PubMed
-
- Linette, G.P., M.J. Grusby, S.M. Hedrick, T.H. Hansen, L.H. Glimcher, and S.J. Korsmeyer. 1994. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1:197–205. - PubMed
-
- Singer, A., R. Bosselut, and A. Bhandoola. 1999. Signals involved in CD4/CD8 lineage commitment: current concepts and potential mechanisms. Semin. Immunol. 11:273–281. - PubMed
-
- Rothenberg, E.V. 1992. The development of functionally responsive T cells. Adv. Immunol. 51:85–214. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

