Amassin, an olfactomedin protein, mediates the massive intercellular adhesion of sea urchin coelomocytes
- PMID: 12591917
- PMCID: PMC2173732
- DOI: 10.1083/jcb.200210053
Amassin, an olfactomedin protein, mediates the massive intercellular adhesion of sea urchin coelomocytes
Abstract
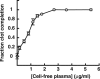
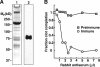
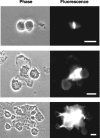
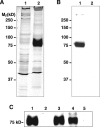
Sea urchins have a fluid-filled body cavity, the coelom, containing four types of immunocytes called coelomocytes. Within minutes after coelomic fluid is removed from the body cavity, a massive cell-cell adhesion of coelomocytes occurs. This event is referred to as clotting. Clotting is thought to be a defense mechanism against loss of coelomic fluid if the body wall is punctured, and it may also function in the cellular encapsulation of foreign material and microbes. Here we show that this intercoelomocyte adhesion is mediated by amassin, a coelomic plasma protein with a relative molecular mass (Mr) of 75 kD. Amassin forms large disulfide-bonded aggregates that adhere coelomocytes to each other. One half of the amassin protein comprises an olfactomedin (OLF) domain. Structural predictions show that amassin and other OLF domain-containing vertebrate proteins share a common architecture. This suggests that other proteins of the OLF family may function in intercellular adhesion. These findings are the first to demonstrate a function for a protein of the OLF family.
Figures




References
-
- Barembaum, M., T.A. Moreno, C. LaBonne, J. Sechrist, and M. Bronner-Fraser. 2000. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nat. Cell Biol. 2:219–225. - PubMed
-
- Bertheussen, K., and R. Seijelid. 1978. Echinoid phagocytes in vitro. Exp. Cell Res. 111:401–412. - PubMed
-
- Bookhout, C.G., and N.D. Greenburg. 1940. Cell types and clotting reactions in the echinoid, Mellita quinquiesperforata Biol. Bull. 79:309–320.
-
- Boolootian, R.A., and A.C. Giese. 1959. Clotting of echinoderm coelomic fluid. J. Exp. Zool. 140:207–229. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

