A mannosyl transferase required for lipopolysaccharide inner core assembly in Rhizobium leguminosarum. Purification, substrate specificity, and expression in Salmonella waaC mutants
- PMID: 12591937
- PMCID: PMC2552394
- DOI: 10.1074/jbc.M301255200
A mannosyl transferase required for lipopolysaccharide inner core assembly in Rhizobium leguminosarum. Purification, substrate specificity, and expression in Salmonella waaC mutants
Abstract
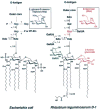
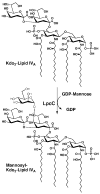
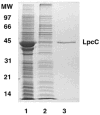
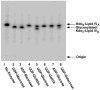
The lipopolysaccharide (LPS) core domain of Gram-negative bacteria plays an important role in outer membrane stability and host interactions. Little is known about the biochemical properties of the glycosyltransferases that assemble the LPS core. We now report the purification and characterization of the Rhizobium leguminosarum mannosyl transferase LpcC, which adds a mannose unit to the inner 3-deoxy-d-manno-octulosonic acid (Kdo) moiety of the LPS precursor, Kdo(2)-lipid IV(A). LpcC containing an N-terminal His(6) tag was assayed using GDP-mannose as the donor and Kdo(2)-[4'-(32)P]lipid IV(A) as the acceptor and was purified to near homogeneity. Sequencing of the N terminus confirmed that the purified enzyme is the lpcC gene product. Mild acid hydrolysis of the glycolipid generated in vitro by pure LpcC showed that the mannosylation occurs on the inner Kdo residue of Kdo(2)-[4'-(32)P]lipid IV(A). A lipid acceptor substrate containing two Kdo moieties is required by LpcC, since no activity is seen with lipid IV(A) or Kdo-lipid IV(A). The purified enzyme can use GDP-mannose or, to a lesser extent, ADP-mannose (both of which have the alpha-anomeric configuration) for the glycosylation of Kdo(2)-[4'-(32)P]lipid IV(A). Little or no activity is seen with ADP-glucose, UDP-glucose, UDP-GlcNAc, or UDP-galactose. A Salmonella typhimurium waaC mutant, which lacks the enzyme for incorporating the inner l-glycero-d-manno-heptose moiety of LPS, regains LPS with O-antigen when complemented with lpcC. An Escherichia coli heptose-less waaC-waaF deletion mutant expressing the R. leguminosarum lpcC gene likewise generates a hybrid LPS species consisting of Kdo(2)-lipid A plus a single mannose residue. Our results demonstrate that heterologous lpcC expression can be used to modify the structure of the Salmonella and E. coli LPS cores in living cells.
Figures




Similar articles
-
Relaxed sugar donor selectivity of a Sinorhizobium meliloti ortholog of the Rhizobium leguminosarum mannosyl transferase LpcC. Role of the lipopolysaccharide core in symbiosis of Rhizobiaceae with plants.J Biol Chem. 2003 May 2;278(18):16365-71. doi: 10.1074/jbc.M301256200. Epub 2003 Feb 17. J Biol Chem. 2003. PMID: 12591936 Free PMC article.
-
Lipopolysaccharide core glycosylation in Rhizobium leguminosarum. An unusual mannosyl transferase resembling the heptosyl transferase I of Escherichia coli.J Biol Chem. 1996 Dec 13;271(50):32119-25. J Biol Chem. 1996. PMID: 8943265
-
Cloning and overexpression of glycosyltransferases that generate the lipopolysaccharide core of Rhizobium leguminosarum.J Biol Chem. 1998 Oct 9;273(41):26432-40. doi: 10.1074/jbc.273.41.26432. J Biol Chem. 1998. PMID: 9756877
-
Lipopolysaccharide biosynthesis in Rhizobium leguminosarum. Novel enzymes that process precursors containing 3-deoxy-D-manno-octulosonic acid.J Biol Chem. 1996 Dec 13;271(50):32112-8. J Biol Chem. 1996. PMID: 8943264
-
LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL.Science. 1964 Aug 21;145(3634):783-9. doi: 10.1126/science.145.3634.783. Science. 1964. PMID: 14163315 Review.
Cited by
-
Expression cloning and biochemical characterization of a Rhizobium leguminosarum lipid A 1-phosphatase.J Biol Chem. 2003 Oct 10;278(41):39269-79. doi: 10.1074/jbc.M305830200. Epub 2003 Jul 16. J Biol Chem. 2003. PMID: 12869541 Free PMC article.
-
Expression cloning of three Rhizobium leguminosarum lipopolysaccharide core galacturonosyltransferases.J Biol Chem. 2006 May 5;281(18):12865-78. doi: 10.1074/jbc.M513864200. Epub 2006 Feb 23. J Biol Chem. 2006. PMID: 16497674 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2003-2004.Mass Spectrom Rev. 2009 Mar-Apr;28(2):273-361. doi: 10.1002/mas.20192. Mass Spectrom Rev. 2009. PMID: 18825656 Free PMC article. Review.
-
Role of BacA in lipopolysaccharide synthesis, peptide transport, and nodulation by Rhizobium sp. strain NGR234.J Bacteriol. 2011 May;193(9):2218-28. doi: 10.1128/JB.01260-10. Epub 2011 Feb 25. J Bacteriol. 2011. PMID: 21357487 Free PMC article.
-
A two-component Kdo hydrolase in the inner membrane of Francisella novicida.Mol Microbiol. 2010 Nov;78(4):820-36. doi: 10.1111/j.1365-2958.2010.07305.x. Mol Microbiol. 2010. PMID: 20662782 Free PMC article.
References
-
- Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. Marcel Dekker, Inc; New York: 1999.
-
- Raetz CRH. Annu Rev Biochem. 1990;59:129–170. - PubMed
-
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. FASEB J. 1994;8:217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

