Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase
- PMID: 12591946
- PMCID: PMC151349
- DOI: 10.1073/pnas.0534892100
Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase
Abstract
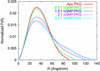
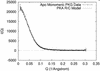
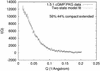
Using small-angle x-ray scattering, we have observed the cGMP-induced elongation of an active, cGMP-dependent, monomeric deletion mutant of cGMP-dependent protein kinase (Delta(1-52)PKG-I beta). On saturation with cGMP, the radius of gyration of Delta(1-52)PKG-I beta increases from 29.4 +/- 0.1 A to 40.1 +/- 0.7 A, and the maximum linear dimension increases from 90 A +/- 10% to 130 A +/- 10%. The elongation is due to a change in the interaction between structured regulatory (R) and catalytic (C) domains. A model of cGMP binding to Delta(1-52)PKG-I beta indicates that elongation of Delta(1-52)PKG-I beta requires binding of cGMP to the low-affinity binding site of the R domain. A comparison with cAMP-dependent protein kinase suggests that both elongation and activation require cGMP binding to both sites; cGMP binding to the low-affinity site therefore seems to be a necessary, but not sufficient, condition for both elongation and activation of Delta(1-52)PKG-I beta. We also predict that there is little or no cooperativity in cGMP binding to the two sites of Delta(1-52)PKG-I beta under the conditions used here. Results obtained by using the Delta(1-52)PKG-I beta monomer indicate that a previously observed elongation of PKG-I alpha is consistent with a pure change in the interaction between the R domain and the C domain, without alteration of the dimerization interaction. This study has revealed important features of molecular mechanisms in the biochemical network describing PKG-I beta activation by cGMP, yielding new insight into ligand activation of cyclic nucleotide-dependent protein kinases, a class of regulatory proteins that is key to many cellular processes.
Figures


References
-
- Francis S H, Corbin J D. Crit Rev Clin Lab Sci. 1999;36:275–328. - PubMed
-
- Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Circ Res. 2000;87:825–830. - PubMed
-
- Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge H R. Trends Biochem Sci. 1997;22:307–312. - PubMed
-
- Kuo J F, Greengard P. J Biol Chem. 1970;245:2493–2498. - PubMed
-
- Yuasa K, Omori K, Yanaka N. J Biol Chem. 2000;275:4897–4905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

