Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine
- PMID: 12591956
- PMCID: PMC151354
- DOI: 10.1073/pnas.0437660100
Oligomeric and polymeric aggregates formed by proteins containing expanded polyglutamine
Abstract
Neurological diseases resulting from proteins containing expanded polyglutamine (polyQ) are characteristically associated with insoluble neuronal inclusions, usually intranuclear, and neuronal death. We describe here oligomeric and polymeric aggregates formed in cells by expanded polyQ. These aggregates are not dissociated by concentrated formic acid, an extremely effective solvent for otherwise insoluble proteins. Perinuclear inclusions formed in cultured cells by expanded polyQ can be completely dissolved in concentrated formic acid, but a soluble protein oligomer containing the expanded polyQ and released by the formic acid is not dissociated to monomer. In Huntington's disease, a formic acid-resistant oligomer is present in cerebral cortex, but not in cerebellum. Cortical nuclei contain a polymeric aggregate of expanded polyQ that is insoluble in formic acid, does not enter polyacrylamide gels, but is retained on filters. This finding shows that the process of polymerization is more advanced in the cerebral cortex than in cultured cells. The resistance of oligomer and polymer to formic acid suggests the participation of covalent bonds in their stabilization.
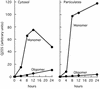
Figures






References
-
- La Spada A R, Wilson E M, Lubahn D B, Harding A E, Fischbeck K H. Nature. 1991;352:77–79. - PubMed
-
- The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- Gusella J F, MacDonald M E. Nat Rev Neurosci. 2000;1:109–115. - PubMed
-
- Eckert R L, Green H. Cell. 1986;46:583–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

