Structure-function analysis of the 3' stem-loop of hepatitis C virus genomic RNA and its role in viral RNA replication
- PMID: 12592007
- PMCID: PMC1370400
- DOI: 10.1261/rna.2144203
Structure-function analysis of the 3' stem-loop of hepatitis C virus genomic RNA and its role in viral RNA replication
Abstract
Previous studies indicate that the 3' terminal 46 nt of the RNA genome of hepatitis C virus (HCV) are highly conserved among different viral strains and essential for RNA replication. Here, we describe a mutational analysis of the 3' terminal hairpin (stem-loop I) that is putatively formed by this sequence and demonstrate its role in replication of the viral RNA. We show that single base substitutions within the 6-nt loop at positions adjacent to the stem abrogate replication of a subgenomic RNA, whereas substitutions in the three apical nucleotides were well tolerated without loss of replication competence. Single point mutations were also well tolerated within the middle section of the duplex, but not at the penultimate nucleotide positions near either end of the stem. However, complementary substitutions at the -19 and -28 positions (from the 3' end) restored replication competence, providing strong evidence for the existence of the structure and its involvement in RNA replication. This was confirmed by rescue of replicating RNAs from mutants containing complementary 10-nt block substitutions at the base of the stem. Each of these RNAs contained an additional U at the 3' terminus. Further experiments indicated a strong preference for U at the 3' terminal position (followed in order by C, A, and G), and a G at the -2 position. These features of stem-loop I are likely to facilitate recognition of the 3' end of the viral RNA by the viral RNA replicase.
Figures







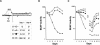

Similar articles
-
3' nontranslated RNA signals required for replication of hepatitis C virus RNA.J Virol. 2003 Mar;77(6):3557-68. doi: 10.1128/jvi.77.6.3557-3568.2003. J Virol. 2003. PMID: 12610131 Free PMC article.
-
The leader RNA of coronavirus mouse hepatitis virus contains an enhancer-like element for subgenomic mRNA transcription.J Virol. 2000 Nov;74(22):10571-80. doi: 10.1128/jvi.74.22.10571-10580.2000. J Virol. 2000. PMID: 11044101 Free PMC article.
-
Role of the 5'-proximal stem-loop structure of the 5' untranslated region in replication and translation of hepatitis C virus RNA.J Virol. 2003 Mar;77(5):3312-8. doi: 10.1128/jvi.77.5.3312-3318.2003. J Virol. 2003. PMID: 12584356 Free PMC article.
-
[Structure and function of the non-coding regions of hepatitis C viral RNA].Postepy Biochem. 2006;52(1):62-71. Postepy Biochem. 2006. PMID: 16869303 Review. Polish.
-
Form confers function: Case of the 3'X region of the hepatitis C virus genome.World J Gastroenterol. 2018 Aug 14;24(30):3374-3383. doi: 10.3748/wjg.v24.i30.3374. World J Gastroenterol. 2018. PMID: 30122877 Free PMC article. Review.
Cited by
-
A comparative method for finding and folding RNA secondary structures within protein-coding regions.Nucleic Acids Res. 2004 Sep 24;32(16):4925-36. doi: 10.1093/nar/gkh839. Print 2004. Nucleic Acids Res. 2004. PMID: 15448187 Free PMC article.
-
Complete nucleotide sequence of a strawberry isolate of Beet pseudoyellows virus.Virus Genes. 2004 Apr;28(3):239-46. doi: 10.1023/b:viru.0000025771.48128.f8. Virus Genes. 2004. PMID: 15266105
-
Cis-acting RNA elements in human and animal plus-strand RNA viruses.Biochim Biophys Acta. 2009 Sep-Oct;1789(9-10):495-517. doi: 10.1016/j.bbagrm.2009.09.007. Epub 2009 Sep 23. Biochim Biophys Acta. 2009. PMID: 19781674 Free PMC article. Review.
-
Nucleolin interacts with the feline calicivirus 3' untranslated region and the protease-polymerase NS6 and NS7 proteins, playing a role in virus replication.J Virol. 2011 Aug;85(16):8056-68. doi: 10.1128/JVI.01878-10. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680514 Free PMC article.
-
cis-Acting RNA elements in the hepatitis C virus RNA genome.Virus Res. 2015 Aug 3;206:90-8. doi: 10.1016/j.virusres.2014.12.029. Epub 2015 Jan 7. Virus Res. 2015. PMID: 25576644 Free PMC article. Review.
References
-
- Alter, M.J., Mast, E.E., Moyer, L.A., and Margolis, H.S. 1998. Hepatitis C. Infect. Dis. Clin. North Am. 12: 13–26. - PubMed
-
- Blight, K.J., Kolykhalov, A.A., Reed, K.E., Agapov, E.V., and Rice, C.M. 1998. Molecular virology of hepatitis C virus: An update with respect to potential antiviral targets. Antivir. Ther. 3: 71–81. - PubMed
-
- Bukh, J., Miller, R.H., and Purcell, R.H. 1995. Genetic heterogeneity of hepatitis C virus: Quasispecies and genotypes. Semin. Liv. Dis. 15: 41–63. - PubMed
-
- Choo, Q.-L., Kuo, G., Weiner, A.J., Overby, L.R., Bradley, D.W., and Houghton, M. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
