Purification of correctly oxidized MHC class I heavy-chain molecules under denaturing conditions: a novel strategy exploiting disulfide assisted protein folding
- PMID: 12592025
- PMCID: PMC2312438
- DOI: 10.1110/ps.0233003
Purification of correctly oxidized MHC class I heavy-chain molecules under denaturing conditions: a novel strategy exploiting disulfide assisted protein folding
Abstract
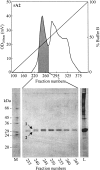
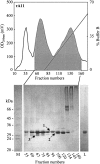
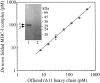
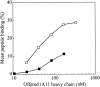
The aim of this study has been to develop a strategy for purifying correctly oxidized denatured major histocompability complex class I (MHC-I) heavy-chain molecules, which on dilution, fold efficiently and become functional. Expression of heavy-chain molecules in bacteria results in the formation of insoluble cellular inclusion bodies, which must be solubilized under denaturing conditions. Their subsequent purification and refolding is complicated by the fact that (1). correct folding can only take place in combined presence of beta(2)-microglobulin and a binding peptide; and (2). optimal in vitro conditions for disulfide bond formation ( approximately pH 8) and peptide binding ( approximately pH 6.6) are far from complementary. Here we present a two-step strategy, which relies on uncoupling the events of disulfide bond formation and peptide binding. In the first phase, heavy-chain molecules with correct disulfide bonding are formed under non-reducing denaturing conditions and separated from scrambled disulfide bond forms by hydrophobic interaction chromatography. In the second step, rapid refolding of the oxidized heavy chains is afforded by disulfide bond-assisted folding in the presence of beta(2)-microglobulin and a specific peptide. Under conditions optimized for peptide binding, refolding and simultaneous peptide binding of the correctly oxidized heavy chain was much more efficient than that of the fully reduced molecule.
Figures



Similar articles
-
Efficient assembly of recombinant major histocompatibility complex class I molecules with preformed disulfide bonds.Eur J Immunol. 2001 Oct;31(10):2986-96. doi: 10.1002/1521-4141(2001010)31:10<2986::aid-immu2986>3.0.co;2-r. Eur J Immunol. 2001. PMID: 11592075
-
Diverse approaches to isolate HLA class I molecules from bacterial inclusion bodies, forming heterotrimeric complexes.Protein Expr Purif. 2025 Jul;231:106713. doi: 10.1016/j.pep.2025.106713. Epub 2025 Mar 26. Protein Expr Purif. 2025. PMID: 40154903
-
A single-chain fusion molecule consisting of peptide, major histocompatibility gene complex class I heavy chain and beta2-microglobulin can fold partially correctly, but binds peptide inefficiently.Scand J Immunol. 1999 Oct;50(4):355-62. doi: 10.1046/j.1365-3083.1999.00601.x. Scand J Immunol. 1999. PMID: 10520174
-
Folding and Purification of Insoluble (Inclusion Body) Proteins from Escherichia coli.Curr Protoc Protein Sci. 2014 Nov 3;78:6.5.1-6.5.30. doi: 10.1002/0471140864.ps0605s78. Curr Protoc Protein Sci. 2014. PMID: 25367010 Review.
-
Thiol oxidation and reduction in MHC-restricted antigen processing and presentation.Immunol Res. 1999;19(2-3):191-200. doi: 10.1007/BF02786487. Immunol Res. 1999. PMID: 10493173 Review.
Cited by
-
The peptide-binding specificity of HLA-A*3001 demonstrates membership of the HLA-A3 supertype.Immunogenetics. 2008 Nov;60(11):633-43. doi: 10.1007/s00251-008-0317-z. Epub 2008 Sep 4. Immunogenetics. 2008. PMID: 18769915
-
Porcine major histocompatibility complex (MHC) class I molecules and analysis of their peptide-binding specificities.Immunogenetics. 2011 Dec;63(12):821-34. doi: 10.1007/s00251-011-0555-3. Epub 2011 Jul 8. Immunogenetics. 2011. PMID: 21739336 Free PMC article.
-
Structural principles of peptide-centric chimeric antigen receptor recognition guide therapeutic expansion.Sci Immunol. 2023 Dec;8(90):eadj5792. doi: 10.1126/sciimmunol.adj5792. Epub 2023 Dec 1. Sci Immunol. 2023. PMID: 38039376 Free PMC article.
-
One-pot, mix-and-read peptide-MHC tetramers.PLoS One. 2008 Feb 27;3(2):e1678. doi: 10.1371/journal.pone.0001678. PLoS One. 2008. PMID: 18301755 Free PMC article.
-
Real-time, high-throughput measurements of peptide-MHC-I dissociation using a scintillation proximity assay.J Immunol Methods. 2011 Nov 30;374(1-2):5-12. doi: 10.1016/j.jim.2010.10.012. Epub 2010 Oct 31. J Immunol Methods. 2011. PMID: 21044632 Free PMC article.
References
-
- Altman, J.D., Moss, P.A., Goulder, P.J., Barouch, D.H., McHeyzer-Williams, M.G., Bell, J.I., McMichael, A.J., and Davis, M.M. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274 94–106. - PubMed
-
- Bernard, A. and Payton, M. 2002. Fermentation and growth of Escherichia coli for optimal protein production. In Current protocols of protein science (eds. J.E. Coligan et al.), unit 5.3. John Wiley & Sons, New York. - PubMed
-
- Buus, S. 1999. Description and prediction of peptide-MHC binding: The "human MHC project." Curr. Opin. Immunol. 11 209–213. - PubMed
-
- Buus, S., Stryhn, A., Winther, K., Kirkby, N., and Pedersen, L.Ø. 1995. Receptor-ligand interactions measured by an improved spun column chromatography technique: A high efficiency and high throughput size separation method. Biochim. Biophys. Acta. 1243 453–460. - PubMed
-
- Chen, M., and Christen, P. 1997. Removal of chromosomal DNA by Mg2+ in the lysis buffer: An improved lysis protocol for preparing Escherichia coli whole-cell lysates for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 246 263–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

