Reinvestigation of the proposed folding and self-association of the Neuropeptide Head Activator
- PMID: 12592026
- PMCID: PMC2312456
- DOI: 10.1110/ps.0232103
Reinvestigation of the proposed folding and self-association of the Neuropeptide Head Activator
Abstract
The Neuropeptide Head Activator (HA), pGlu-Pro-Pro-Gly-Gly-Ser-Lys-Val-Ile-Leu-Phe (pGlu is pyroglutamic acid), is involved in head-specific growth and differentiation processes in the freshwater coelenterate Hydra attenuata. Peptides of identical sequence have also been isolated from higher-organism tissues such as human and bovine hypothalamus. Early studies by molecular sieve chromatography suggested that HA dimerizes with high affinity (K(d) approximately 1 nM). This dimerization was proposed to occur via antiparallel beta-sheet formation between the Lys(7)-Phe(11) segments in each HA molecule. We conducted biophysical studies on synthetic HA in order to gain insight into its structure and aggregation tendencies. We found by analytical ultracentrifugation that HA is monomeric at low millimolar concentrations. Studies by (1)H-NMR revealed that HA did not adopt any significant secondary structure in solution. We found no NOEs that would support the proposed dimer structure. We probed the propensity of the Lys(7)-Phe(11) fragment to form antiparallel beta-sheet by designing peptides in which two such fragments are joined by a two-residue linker. These peptides were intended to form stable beta-hairpin structures with cross-strand interactions that mimic those of the proposed HA dimer interface. We found that the HA-derived fragments may be induced to form intramolecular beta-sheet, albeit only weakly, when linked by the highly beta-hairpin-promoting D-Pro-Gly turn, but not when linked by the more flexible Gly-Gly unit. These findings suggest that the postulated mode of HA dimerization and the proposed propensity of the molecule to form discrete aggregates with high affinity are incorrect.
Figures




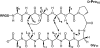
Similar articles
-
Peptide hairpins with strand segments containing alpha- and beta-amino acid residues: cross-strand aromatic interactions of facing Phe residues.Biopolymers. 2005;80(6):787-99. doi: 10.1002/bip.20294. Biopolymers. 2005. PMID: 15895435 Free PMC article.
-
The neuropeptide head activator loses its biological acitivity by dimerization.EMBO J. 1986 Aug;5(8):1825-9. doi: 10.1002/j.1460-2075.1986.tb04433.x. EMBO J. 1986. PMID: 2577954 Free PMC article.
-
Hydra head activator peptide has trophic activity for eukaryotic neurons.Brain Res Dev Brain Res. 1992 Jul 24;68(1):97-102. doi: 10.1016/0165-3806(92)90251-q. Brain Res Dev Brain Res. 1992. PMID: 1521328
-
Design of monomeric water-soluble β-hairpin and β-sheet peptides.Methods Mol Biol. 2014;1216:15-52. doi: 10.1007/978-1-4939-1486-9_2. Methods Mol Biol. 2014. PMID: 25213409 Review.
-
Role of the neuropeptide head activator for growth and development in hydra and mammals.Development. 1989;107 Suppl:99-107. doi: 10.1242/dev.107.Supplement.99. Development. 1989. PMID: 2699861 Review.
Cited by
-
Enhanced therapeutic window for antimicrobial Pept-ins by investigating their structure-activity relationship.PLoS One. 2023 Mar 31;18(3):e0283674. doi: 10.1371/journal.pone.0283674. eCollection 2023. PLoS One. 2023. PMID: 37000776 Free PMC article.
References
-
- Arndt, K.M., Pelletier, J.N., Muller, K.M., Alber, T., Michnick, S.W., and Pluckthun, A. 2000. A heterodimeric coiled-coil peptide pair selected in vivo from a designed library-versus-library ensemble. J. Mol. Biol. 295 627–639. - PubMed
-
- Bax, A. and Davies, D.G. 1985. MLEV-17 based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 65 355–360.
-
- Birr, C., Zachmann, B., Bodenmuller, H., and Schaller, H.C. 1981. Synthesis of a new neuropeptide, the head activator from hydra. FEBS Lett. 131 317–321. - PubMed
-
- Bodenmuller, H. and Schaller, H.C. 1981. Conserved amino acid sequence of a neuropeptide, the head activator, from coelenterates to humans. Nature 293 579–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

