Bacterial enterotoxins are associated with resistance to colon cancer
- PMID: 12594332
- PMCID: PMC151403
- DOI: 10.1073/pnas.0434905100
Bacterial enterotoxins are associated with resistance to colon cancer
Abstract
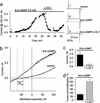
One half million patients suffer from colorectal cancer in industrialized nations, yet this disease exhibits a low incidence in under-developed countries. This geographic imbalance suggests an environmental contribution to the resistance of endemic populations to intestinal neoplasia. A common epidemiological characteristic of these colon cancer-spared regions is the prevalence of enterotoxigenic bacteria associated with diarrheal disease. Here, a bacterial heat-stable enterotoxin was demonstrated to suppress colon cancer cell proliferation by a guanylyl cyclase C-mediated signaling cascade. The heat-stable enterotoxin suppressed proliferation by increasing intracellular cGMP, an effect mimicked by the cell-permeant analog 8-br-cGMP. The antiproliferative effects of the enterotoxin and 8-br-cGMP were reversed by L-cis-diltiazem, a cyclic nucleotide-gated channel inhibitor, as well as by removal of extracellular Ca(2+), or chelation of intracellular Ca(2+). In fact, both the enterotoxin and 8-br-cGMP induced an L-cis-diltiazem-sensitive conductance, promoting Ca(2+) influx and inhibition of DNA synthesis in colon cancer cells. Induction of this previously unrecognized antiproliferative signaling pathway by bacterial enterotoxin could contribute to the resistance of endemic populations to intestinal neoplasia, and offers a paradigm for targeted prevention and therapy of primary and metastatic colorectal cancer.
Figures



Comment in
-
Diarrhea or colorectal cancer: can bacterial toxins serve as a treatment for colon cancer?Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3018-20. doi: 10.1073/pnas.0730484100. Epub 2003 Mar 11. Proc Natl Acad Sci U S A. 2003. PMID: 12631696 Free PMC article. No abstract available.
References
-
- Ferlay J, Bray F, Pisani P, Parkin D M. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. Lyon: International Agency for Research on Cancer; 2001.
-
- Hill D R, Pearson R D. Ann Intern Med. 1988;108:839–852. - PubMed
-
- Centers for Disease Control and Prevention. Health Information for International Travel 1999–2000. Atlanta: Department of Health and Human Services; 2001.
-
- Schulz S, Green C K, Yuen P S, Garbers D L. Cell. 1990;63:941–948. - PubMed
-
- Hughes J M, Murad F, Chang B, Guerrant R L. Nature. 1978;271:755–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

