Primed peritoneal B lymphocytes are sufficient to transfer protection against Brugia pahangi infection in mice
- PMID: 12595454
- PMCID: PMC148870
- DOI: 10.1128/IAI.71.3.1370-1378.2003
Primed peritoneal B lymphocytes are sufficient to transfer protection against Brugia pahangi infection in mice
Abstract
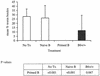
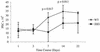
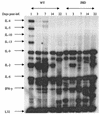
Lymphatic filariasis is a tropical disease caused by the nematode parasites Wuchereria bancrofti and Brugia malayi. Whereas the protective potential of T lymphocytes in filarial infection is well documented, investigation of the role of B lymphocytes in antifilarial immunity has been neglected. In this communication, we examine the role of B lymphocytes in antifilarial immunity, using Brugia pahangi infections in the murine peritoneal cavity as a model. We find that B lymphocytes are required for clearance of primary and challenge infections with B. pahangi third-stage larvae (L3). We assessed the protective potential of peritoneal B lymphocytes by adoptive transfer experiments. Primed but not naïve peritoneal B cells from wild-type mice that had been immunized with B. pahangi L3 protected athymic recipients from challenge infection. We evaluated possible mechanisms by which B cells mediate protection. Comparisons of cytokine mRNA expression between B-lymphocyte-deficient and immunocompetent mice following B. pahangi infection suggest that B cells are required for the early production of Th2-type cytokines by peritoneal cells. In addition, B-cell-deficient mice demonstrate a defect in inflammatory cell recruitment to the peritoneal cavity following B. pahangi infection. The data demonstrate a critical role of B lymphocytes in antifilarial immunity in naïve mice and in the memory response in primed mice.
Figures



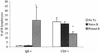
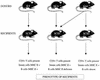

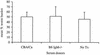
References
-
- Al-Qaoud, K. M., E. Pearlman, T. Hartung, J. Klukowski, B. Fleischer, and A. Hoerauf. 2000. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 12:899-908. - PubMed
-
- Anonymous. 2001. Lymphatic filariasis. Wkly. Epidemiol. Rec. 76:149-154. - PubMed
-
- Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. - PMC - PubMed
-
- Bancroft, A. J., R. K. Grencis, K. J. Else, and E. Devaney. 1994. The role of CD4 cells in protective immunity to Brugia pahangi. Parasite Immunol. 16:385-387. - PubMed
-
- Bikah, G., J. Carey, J. R. Ciallella, A. Tarakhovsky, and S. Bondada. 1996. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science 274:1906-1909. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

