Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice
- PMID: 12595471
- PMCID: PMC148884
- DOI: 10.1128/IAI.71.3.1520-1526.2003
Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice
Abstract
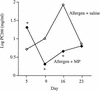
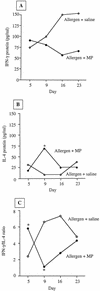
Airway mycoplasma infection may be associated with asthma pathophysiology. However, the direct effects of mycoplasma infection on asthma remain unknown. Using a murine allergic-asthma model, we evaluated the effects of different timing of airway Mycoplasma pneumoniae infection on bronchial hyperresponsiveness (BHR), lung inflammation, and the protein levels of Th1 (gamma interferon [IFN-gamma]) and Th2 (interleukin 4 [IL-4]) cytokines in bronchoalveolar lavage fluid. When mycoplasma infection occurred 3 days before allergen (ovalbumin) sensitization and challenge, the infection reduced the BHR and inflammatory-cell influx into the lung. This was accompanied by a significant induction of Th1 responses (increased IFN-gamma and decreased IL-4 production). Conversely, when mycoplasma infection occurred 2 days after allergen sensitization and challenge, the infection initially caused a temporary reduction of BHR and then increased BHR, lung inflammation, and IL-4 levels. Our data suggest that mycoplasma infection could modulate both physiological and immunological responses in the murine asthma model. Our animal models may also provide a new means to understand the role of infection in asthma pathogenesis and give evidence for the asthma hygiene hypothesis.
Figures




References
-
- Busse, W. W., and R. F. Lemanske, Jr. 2001. Asthma. N. Engl. J. Med. 344:350-356. - PubMed
-
- Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

