Bacterial [Cu,Zn]-cofactored superoxide dismutase protects opsonized, encapsulated Neisseria meningitidis from phagocytosis by human monocytes/macrophages
- PMID: 12595487
- PMCID: PMC148830
- DOI: 10.1128/IAI.71.3.1604-1607.2003
Bacterial [Cu,Zn]-cofactored superoxide dismutase protects opsonized, encapsulated Neisseria meningitidis from phagocytosis by human monocytes/macrophages
Abstract
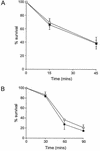
Superoxide dismutase cofactored by copper and zinc ([Cu,Zn]-SOD) contributes to the protection of opsonized serogroup B Neisseria meningitidis against phagocytosis by human monocytes/macrophages, with sodC mutant organisms being endocytosed in significantly higher numbers than are wild-type organisms. The influence of [Cu,Zn]-SOD was found to be exerted at the stage of phagocytosis, rather than at earlier (modulating surface association) or later (intracellular killing) stages.
Figures
References
-
- Achtman, M., M. Neibert, B. Crowe, W. Strittmatter, B. Kusecek, E. Weyse, M. Walsh, B. Slawig, G. Morelli, and A. Moll. 1988. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J. Exp. Med. 168:507-525. - PMC - PubMed
-
- Battistoni, A., F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio, and P. Valenti. 2000. Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect. Immun. 68:30-37. - PMC - PubMed
-
- Beck, B. L., L. B. Tabatabai, and J. E. Mayfield. 1990. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry 29:372-376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

