Efficient cloning and engineering of entire mitochondrial genomes in Escherichia coli and transfer into transcriptionally active mitochondria
- PMID: 12595548
- PMCID: PMC149821
- DOI: 10.1093/nar/gkg228
Efficient cloning and engineering of entire mitochondrial genomes in Escherichia coli and transfer into transcriptionally active mitochondria
Abstract
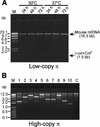
We have devised an efficient method for replicating and stably maintaining entire mitochondrial genomes in Escherichia coli and have shown that we can engineer these mitochondrial DNA (mtDNA) genome clones using standard molecular biological techniques. In general, we accomplish this by inserting an E.coli replication origin and selectable marker into isolated, circular mtDNA at random locations using an in vitro transposition reaction and then transforming the modified genomes into E.coli. We tested this approach by cloning the 16.3 kb mouse mitochondrial genome and found that the resulting clones could be engineered and faithfully maintained when we used E.coli hosts that replicated them at moderately low copy numbers. When these recombinant mtDNAs were replicated at high copy numbers, however, mtDNA sequences were partially or fully deleted from the original clone. We successfully electroporated recombinant mouse mitochondrial genomes into isolated mouse mitochondria devoid of their own DNA and detected robust in organello RNA synthesis by RT-PCR. This approach for modifying mtDNA and subsequent in organello analysis of the recombinant genomes offers an attractive experimental system for studying many aspects of vertebrate mitochondrial gene expression and is a first step towards true in vivo engineering of mammalian mitochondrial genomes.
Figures



References
-
- Saraste M. (1999) Oxidative phosphorylation at the fin de siecle. Science, 283, 1488–1493. - PubMed
-
- Green D.R. and Reed,J.C. (1998) Mitochondria and apoptosis. Science, 281, 1309–1312. - PubMed
-
- Gray M.W., Burger,G. and Lang,B.F. (1999) Mitochondrial evolution. Science, 283, 1476–1481. - PubMed
-
- Wallace D.C. (1999) Mitochondrial diseases in man and mouse. Science, 283, 1482–1488. - PubMed
-
- Johnston S.A., Anziano,P.Q., Shark,K., Sanford,J.C. and Butow,R.A. (1988) Mitochondrial transformation in yeast by bombardment with microprojectiles. Science, 240, 1538–1541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

