Identification of the SRC pyrimidine-binding protein (SPy) as hnRNP K: implications in the regulation of SRC1A transcription
- PMID: 12595559
- PMCID: PMC149839
- DOI: 10.1093/nar/gkg246
Identification of the SRC pyrimidine-binding protein (SPy) as hnRNP K: implications in the regulation of SRC1A transcription
Abstract
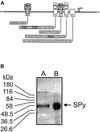
The human SRC gene encodes pp60(c-src), a non-receptor tyrosine kinase involved in numerous signaling pathways. Activation or overexpression of c-Src has also been linked to a number of important human cancers. Transcription of the SRC gene is complex and regulated by two closely linked but highly dissimilar promoters, each associated with its own distinct non-coding exon. In many tissues SRC expression is regulated by the housekeeping-like SRC1A promoter. In addition to other regulatory elements, three substantial polypurine:polypyrimidine (TC) tracts within this promoter are required for full transcriptional activity. Previously, we described an unusual factor called SRC pyrimidine-binding protein (SPy) that could bind to two of these TC tracts in their double-stranded form, but was also capable of interacting with higher affinity to all three pyrimidine tracts in their single-stranded form. Mutations in the TC tracts, which abolished the ability of SPy to interact with its double-stranded DNA target, significantly reduced SRC1A promoter activity, especially in concert with mutations in critical Sp1 binding sites. Here we expand upon our characterization of this interesting factor and describe the purification of SPy from human SW620 colon cancer cells using a DNA affinity-based approach. Subsequent in-gel tryptic digestion of purified SPy followed by MALDI-TOF mass spectrometric analysis identified SPy as heterogeneous nuclear ribonucleoprotein K (hnRNP K), a known nucleic-acid binding protein implicated in various aspects of gene expression including transcription. These data provide new insights into the double- and single-stranded DNA-binding specificity, as well as functional properties of hnRNP K, and suggest that hnRNP K is a critical component of SRC1A transcriptional processes.
Figures



Similar articles
-
Transcription of the human c-Src promoter is dependent on Sp1, a novel pyrimidine binding factor SPy, and can be inhibited by triplex-forming oligonucleotides.J Biol Chem. 2000 Jan 14;275(2):847-54. doi: 10.1074/jbc.275.2.847. J Biol Chem. 2000. PMID: 10625617
-
Transcriptional regulation of heterogeneous nuclear ribonucleoprotein K gene expression.Biochimie. 2015 Feb;109:27-35. doi: 10.1016/j.biochi.2014.12.002. Epub 2014 Dec 10. Biochimie. 2015. PMID: 25497182 Free PMC article.
-
An alternative, human SRC promoter and its regulation by hepatic nuclear factor-1alpha.J Biol Chem. 2000 Dec 1;275(48):37604-11. doi: 10.1074/jbc.M004882200. J Biol Chem. 2000. PMID: 10978326
-
Diverse molecular interactions of the hnRNP K protein.FEBS Lett. 1997 Feb 17;403(2):113-5. doi: 10.1016/s0014-5793(97)00041-0. FEBS Lett. 1997. PMID: 9042948 Review.
-
Aberrant hnRNP K expression: All roads lead to cancer.Cell Cycle. 2016 Jun 17;15(12):1552-7. doi: 10.1080/15384101.2016.1164372. Cell Cycle. 2016. PMID: 27049467 Free PMC article. Review.
Cited by
-
Heterogeneous nuclear ribonucleoprotein (HnRNP) K genome-wide binding survey reveals its role in regulating 3'-end RNA processing and transcription termination at the early growth response 1 (EGR1) gene through XRN2 exonuclease.J Biol Chem. 2013 Aug 23;288(34):24788-98. doi: 10.1074/jbc.M113.496679. Epub 2013 Jul 15. J Biol Chem. 2013. PMID: 23857582 Free PMC article.
-
G-quadruplex formation within the promoter of the KRAS proto-oncogene and its effect on transcription.Nucleic Acids Res. 2006 May 10;34(9):2536-49. doi: 10.1093/nar/gkl286. Print 2006. Nucleic Acids Res. 2006. PMID: 16687659 Free PMC article.
-
LncRNA CRLM1 inhibits apoptosis and promotes metastasis through transcriptional regulation cooperated with hnRNPK in colorectal cancer.Cell Biosci. 2022 Jul 30;12(1):120. doi: 10.1186/s13578-022-00849-9. Cell Biosci. 2022. PMID: 35907898 Free PMC article.
-
Regulation of the p53 expression profile by hnRNP K under stress conditions.RNA Biol. 2020 Oct;17(10):1402-1415. doi: 10.1080/15476286.2020.1771944. Epub 2020 May 29. RNA Biol. 2020. PMID: 32449427 Free PMC article.
-
Role and molecular mechanism of heterogeneous nuclear ribonucleoprotein K in tumor development and progression.Biomed Rep. 2016 Jun;4(6):657-663. doi: 10.3892/br.2016.642. Epub 2016 Mar 29. Biomed Rep. 2016. PMID: 27284403 Free PMC article.
References
-
- Abram C.L. and Courtneidge,S.A. (2000) Src family tyrosine kinases and growth factor signaling. Exp. Cell Res., 254, 1–13. - PubMed
-
- Biscardi J.S., Tice,D.A. and Parsons,S.J. (1999) c-Src, receptor tyrosine kinases and human cancer. Adv. Cancer Res., 76, 61–119. - PubMed
-
- Brown M.T. and Cooper,J.A. (1996) Regulation, substrates and functions of src. Biochim. Biophys. Acta, 1287, 121–149. - PubMed
-
- Thomas S.M. and Brugge,J.S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol., 13, 513–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

