Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways
- PMID: 12598315
- PMCID: PMC1868104
- DOI: 10.1016/S0002-9440(10)63877-1
Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways
Abstract
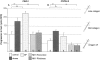
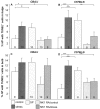
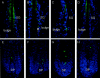
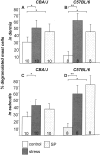
It has been much disputed whether or not stress can cause hair loss (telogen effluvium) in a clinically relevant manner. Despite the paramount psychosocial importance of hair in human society, this central, yet enigmatic and controversial problem of clinically applied stress research has not been systematically studied in appropriate animal models. We now show that psychoemotional stress indeed alters actual hair follicle (HF) cycling in vivo, ie, prematurely terminates the normal duration of active hair growth (anagen) in mice. Further, inflammatory events deleterious to the HF are present in the HF environment of stressed mice (perifollicular macrophage cluster, excessive mast cell activation). This provides the first solid pathophysiological mechanism for how stress may actually cause telogen effluvium, ie, by hair cycle manipulation and neuroimmunological events that combine to terminate anagen. Furthermore, we show that most of these hair growth-inhibitory effects of stress can be reproduced by the proteotypic stress-related neuropeptide substance P in nonstressed mice, and can be counteracted effectively by co-administration of a specific substance P receptor antagonist in stressed mice. This offers the first convincing rationale how stress-induced hair loss in men may be pharmacologically managed effectively.
Figures



Comment in
-
Stress and the hair follicle: exploring the connections.Am J Pathol. 2003 Mar;162(3):709-12. doi: 10.1016/S0002-9440(10)63866-7. Am J Pathol. 2003. PMID: 12598304 Free PMC article. Review. No abstract available.
Similar articles
-
Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control.Exp Dermatol. 2006 Jan;15(1):1-13. doi: 10.1111/j.0906-6705.2005.00372.x. Exp Dermatol. 2006. PMID: 16364026 Review.
-
Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice.PLoS One. 2013 Apr 26;8(4):e61574. doi: 10.1371/journal.pone.0061574. Print 2013. PLoS One. 2013. PMID: 23637859 Free PMC article.
-
Indications for a 'brain-hair follicle axis (BHA)': inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P.FASEB J. 2001 Nov;15(13):2536-8. doi: 10.1096/fj.00-0699fje. Epub 2001 Sep 17. FASEB J. 2001. PMID: 11641256
-
Activated skin mast cells are involved in murine hair follicle regression (catagen).Lab Invest. 1997 Oct;77(4):319-32. Lab Invest. 1997. PMID: 9354767
-
Epithelial growth control by neurotrophins: leads and lessons from the hair follicle.Prog Brain Res. 2004;146:493-513. doi: 10.1016/S0079-6123(03)46031-7. Prog Brain Res. 2004. PMID: 14699982 Review.
Cited by
-
The role of mast cells in non-ablative laser resurfacing with 1,320 nm neodymium:yttrium-aluminium-garnet laser.Lasers Med Sci. 2010 May;25(3):371-7. doi: 10.1007/s10103-009-0703-2. Epub 2009 Jun 30. Lasers Med Sci. 2010. PMID: 19565311
-
Hair and stress: A pilot study of hair and cytokine balance alteration in healthy young women under major exam stress.PLoS One. 2017 Apr 19;12(4):e0175904. doi: 10.1371/journal.pone.0175904. eCollection 2017. PLoS One. 2017. PMID: 28423056 Free PMC article.
-
Presence of Mast Cells and Mast Cell Degranulation in Scalp Biopsies of Telogen Effluvium.Int J Trichology. 2017 Jan-Mar;9(1):25-29. doi: 10.4103/ijt.ijt_43_16. Int J Trichology. 2017. PMID: 28761261 Free PMC article.
-
Probing the effects of stress mediators on the human hair follicle: substance P holds central position.Am J Pathol. 2007 Dec;171(6):1872-86. doi: 10.2353/ajpath.2007.061206. Epub 2007 Nov 30. Am J Pathol. 2007. PMID: 18055548 Free PMC article.
-
Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss.J Clin Med. 2023 Jan 23;12(3):893. doi: 10.3390/jcm12030893. J Clin Med. 2023. PMID: 36769541 Free PMC article. Review.
References
-
- Selye H: The Physiology and Pathology of Exposure to Stress. 1950:p 727 ACTA Inc. Medical Publishers, Montreal
-
- Trepat L, Petre AJ: ‘Pelada Universal por shock emotiva.’ Semana Méd 1942, 1:65
-
- Whitlock FA: Rook A eds. Psychophysiological aspects of skin disease. Major Problems in Dermatology 1976, vol 8. Saunders, London
-
- York J, Nicholson T, Minors P, Duncan DF: Stressful life events and loss of hair among adult women, a case-control study. Psychol Rep 1998, 82:1044-1046 - PubMed
-
- Garcia-Hernandez MJ, Ruiz-Doblado S, Rodriguez-Pichardo A, Camacho F: Alopecia areata, stress and psychiatric disorders: a review. J Dermatol 1999, 26:625-662 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

