Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder
- PMID: 12598324
- PMCID: PMC1868088
- DOI: 10.1016/S0002-9440(10)63886-2
Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder
Abstract
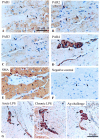
Inflammation underlines all major bladder pathologies and represents a defense reaction to injury involving a mandatory participation of mast cells and sensory nerves. Mast cells are particularly frequent in close proximity to epithelial surfaces where they are strategically located in the bladder and release their mediators in response to inflammation. Tryptase is specifically produced by mast cells and modulates inflammation by activating protease-activated receptors (PARs). We recently found that PAR-4 mRNA is up-regulated in experimental bladder inflammation regardless of the initiating stimulus. Because it has been reported that PAR-1, PAR-2, and PAR-3 may also be involved in the processes of inflammation, we used immunohistochemistry to characterize the expression of all known PARs in normal, acute, and chronic inflamed mouse bladder. We found that all four PARs are present in the control mouse bladder, and follow a unique distribution. All four PARs are co-expressed in the urothelium, whereas PAR-1 and PAR-2 are predominant in the detrusor muscle, and PAR-4 is expressed in peripheral nerves and plexus cell bodies. The strong expression of PARs in the detrusor muscle indicates the need for studies on the role of these receptors in motility whereas the presence of PAR-4 in nerves may indicate its participation in neurogenic inflammation. In addition, PARs are differentially modulated during inflammation. PAR-1 and PAR-2 are down-regulated in acute inflammation whereas PAR-3 and PAR-4 are up-regulated. Bladder fibroblasts were found to present a clear demarcation in PAR expression secondary to acute and chronic inflammation. Our findings provide evidence of participation of PARs in the urinary system, provide a working model for mast cell tryptase signaling in the mouse bladder, and evoke testable hypotheses regarding the roles of PARs in bladder inflammation. It is timely to understand the role of tryptase signaling and PARs in the context of bladder biology.
Figures








References
-
- Okragly A, Niles A, Saban R, Schmidt D, Hoffman R, Warner T, Moon T, Uehling D, Haak-Frendscho M: Elevated tryptase, NGF, NT-3, and GDNF levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 1999, 161:434-441 - PubMed
-
- Hollenberg D, Compton S: International Union of Pharmacology. XXVIII. Proteinase-Activated Receptors. Pharmacol Rev 2002, 54:203-217 - PubMed
-
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD: Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001, 22:146-152 - PubMed
-
- Vergnolle N: Proteinase-activated receptors—novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 2000, 14:257-266 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

