The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions
- PMID: 12598408
- PMCID: PMC1573706
- DOI: 10.1038/sj.bjp.0705100
The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions
Abstract
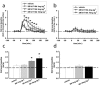
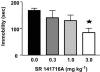
1. In order to explore potential therapeutic implications of cannabinoid antagonists, the effects of the prototypical cannabinoid antagonist SR141716A on monoamine efflux from the medial prefrontal cortex and the nucleus accumbens of the rat were investigated by in vivo microdialysis. 2. SR141716A moderately increased serotonin efflux and concentrations of its metabolite 5-HIAA, both in the medial prefrontal cortex and the nucleus accumbens, and increased norepinephrine, dopamine and their metabolites in the medial prefrontal cortex. In contrast, it had no effect on norepinephrine, dopamine and their metabolites in the nucleus accumbens. 3. At the same doses, SR141716A increased acetylcholine efflux in the medial prefrontal cortex, in agreement with previous studies; contrary to the effects in cortex, SR141716A had no effect on acetylcholine efflux in the nucleus accumbens. 4. The efficacy of SR141716A in the psychostimulant-induced hyperlocomotion and the forced swimming paradigms was also explored in mice. SR141716A attenuated phenylcyclidine- and d-amphetamine-induced hyperlocomotion, without affecting locomotor activity when administered alone, and decreased immobility in the forced swimming test. 5. These results suggest that the cortical selectivity in the release of catecholamines, dopamine in particular, induced by the cannabinoid antagonist SR141716A, its procholinergic properties, together with its mild stimulatory effects on serotonin and norepinephrine efflux make similar compounds unique candidates for the treatment of psychosis, affective and cognitive disorders.
Figures
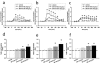



Similar articles
-
Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study.Eur J Neurosci. 2002 Jun;15(11):1795-802. doi: 10.1046/j.1460-9568.2002.02019.x. Eur J Neurosci. 2002. PMID: 12081659
-
Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function.Neuroscience. 1999;91(2):607-20. doi: 10.1016/s0306-4522(98)00675-7. Neuroscience. 1999. PMID: 10366018
-
Cannabinoids negatively modulate striatal glutamate and dopamine release and behavioural output of acute D-amphetamine.Behav Brain Res. 2014 Aug 15;270:261-9. doi: 10.1016/j.bbr.2014.05.029. Epub 2014 May 24. Behav Brain Res. 2014. PMID: 24867330
-
The neuropsychopharmacology of fronto-executive function: monoaminergic modulation.Annu Rev Neurosci. 2009;32:267-87. doi: 10.1146/annurev.neuro.051508.135535. Annu Rev Neurosci. 2009. PMID: 19555290 Free PMC article. Review.
-
Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex.Brain Sci. 2024 Sep 6;14(9):902. doi: 10.3390/brainsci14090902. Brain Sci. 2024. PMID: 39335398 Free PMC article. Review.
Cited by
-
Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels.Front Integr Neurosci. 2013 May 23;7:40. doi: 10.3389/fnint.2013.00040. eCollection 2013. Front Integr Neurosci. 2013. PMID: 23734105 Free PMC article.
-
Synergistic Effect between Maternal Infection and Adolescent Cannabinoid Exposure on Serotonin 5HT1A Receptor Binding in the Hippocampus: Testing the "Two Hit" Hypothesis for the Development of Schizophrenia.ISRN Psychiatry. 2012 Jun 7;2012:451865. doi: 10.5402/2012/451865. Print 2012. ISRN Psychiatry. 2012. PMID: 23738203 Free PMC article.
-
Presynaptic CB(1) cannabinoid receptors control frontocortical serotonin and glutamate release--species differences.Neurochem Int. 2012 Jul;61(2):219-26. doi: 10.1016/j.neuint.2012.05.009. Epub 2012 May 17. Neurochem Int. 2012. PMID: 22609378 Free PMC article.
-
Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality.Psychopharmacology (Berl). 2007 May;192(1):61-70. doi: 10.1007/s00213-006-0689-4. Epub 2007 Feb 6. Psychopharmacology (Berl). 2007. PMID: 17279376
-
Prejunctional and peripheral effects of the cannabinoid CB(1) receptor inverse agonist rimonabant (SR 141716).Naunyn Schmiedebergs Arch Pharmacol. 2008 Oct;378(4):345-69. doi: 10.1007/s00210-008-0327-2. Epub 2008 Jul 25. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18654765 Review.
References
-
- ACQUAS E., PISANU A., MARROCU P., DI CHIARA G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur. J. Pharmacol. 2000;401:179–185. - PubMed
-
- ALONSO R., VOUTSINOS B., FOURNIER M., LABIE C., STEINBERG R., SOUILHAC J., LE FUR G., SOUBRIE P. Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 1999;91:607–620. - PubMed
-
- ARBORELIUS L., NOMIKOS G.G., HACKSELL U., SVENSSON T.H. (R)-8-OH-DPAT preferentially increases dopamine release in rat medial prefrontal cortex. Acta Physiol. Scand. 1993;148:465–466. - PubMed
-
- AUCLAIR N., OTANI S., SOUBRIE P., CREPEL F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000;83:3287–3293. - PubMed
-
- BAXTER L.R., JR, SCHWARTZ J.M., PHELPS M.E., MAZZIOTTA J.C., GUZE B.H., SELIN C.E., GERNER R.H., SUMIDA R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46:243–250. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

