Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2)
- PMID: 12598410
- PMCID: PMC1573691
- DOI: 10.1038/sj.bjp.0705049
Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2)
Abstract
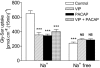
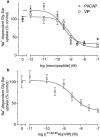
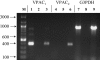
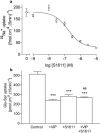
1. Optimal dipeptide and peptidomimetic drug transport across the intestinal mucosal surface is dependent upon the co-operative functional activity of the di/tripeptide transporter hPepT1 and the Na(+)/H(+) exchanger NHE3. The ability of the anti-absorptive enteric neuropeptide VIP (vasoactive intestinal peptide) to modulate dipeptide uptake was determined using human intestinal (Caco-2) epithelial cell monolayers. 2. Uptake of glycylsarcosine (Gly-Sar) across the apical membrane of Caco-2 cell monolayers is inhibited by basolateral exposure to either VIP, pituitary adenylate cyclase-activating polypeptide (PACAP), or the VPAC(1) receptor agonist [(11,22,28)Ala]-VIP. Inhibition of Gly-Sar uptake is observed only in the presence of extracellular Na(+). Reverse-transcription polymerase chain reaction (RT-PCR) demonstrates that VPAC(1) mRNA is expressed in Caco-2 cells whereas VPAC(2) mRNA is not detected. 3. The VIP-induced inhibition of Gly-Sar uptake is abolished in the presence of the protein kinase A (PKA) inhibitor H-89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide.2HCl). 4. (22)Na(+) uptake across the apical membrane is inhibited by the selective NHE3 inhibitor S1611. Experiments with BCECF [2',7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein]-loaded Caco-2 cells demonstrate that VIP reduces the NHE3-dependent recovery of intracellular pH (pH(i)) after dipeptide-induced acidification. Western blot of Caco-2 cell protein demonstrates expression of the NHE regulatory factor NHERF1 (expression of which is thought to be required for PKA-mediated inhibition of NHE3). 5. VIP has no effect on Gly-Sar uptake in the presence of S1611 suggesting that VIP and S1611 both modulate dipeptide uptake via the same mechanism. 6. These observations demonstrate that VIP (and PACAP) modulate activity of the H(+)/dipeptide transporter hPepT1 in a Na(+)-dependent manner consistent with the modulation being indirect through inhibition of NHE3.
Figures


Similar articles
-
Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity.Pflugers Arch. 2002 Oct;445(1):139-46. doi: 10.1007/s00424-002-0910-1. Epub 2002 Aug 9. Pflugers Arch. 2002. PMID: 12397398
-
Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1).J Cell Physiol. 2005 Aug;204(2):604-13. doi: 10.1002/jcp.20337. J Cell Physiol. 2005. PMID: 15754324
-
H/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na/H exchanger.Gastroenterology. 2002 May;122(5):1322-33. doi: 10.1053/gast.2002.32992. Gastroenterology. 2002. PMID: 11984519
-
Molecular and integrative physiology of intestinal peptide transport.Annu Rev Physiol. 2004;66:361-84. doi: 10.1146/annurev.physiol.66.032102.144149. Annu Rev Physiol. 2004. PMID: 14977407 Review.
-
Structure-activity relationship of vasoactive intestinal peptide (VIP): potent agonists and potential clinical applications.Naunyn Schmiedebergs Arch Pharmacol. 2008 Jun;377(4-6):579-90. doi: 10.1007/s00210-007-0232-0. Epub 2008 Jan 3. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18172612 Review.
Cited by
-
H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine.Exp Physiol. 2007 Jul;92(4):603-19. doi: 10.1113/expphysiol.2005.029959. Epub 2007 Apr 27. Exp Physiol. 2007. PMID: 17468205 Free PMC article. Review.
-
The Role of Plasma Membrane Sodium/Hydrogen Exchangers in Gastrointestinal Functions: Proliferation and Differentiation, Fluid/Electrolyte Transport and Barrier Integrity.Front Physiol. 2022 May 18;13:899286. doi: 10.3389/fphys.2022.899286. eCollection 2022. Front Physiol. 2022. PMID: 35665228 Free PMC article. Review.
-
Reciprocal regulation of the primary sodium absorptive pathways in rat intestinal epithelial cells.Am J Physiol Cell Physiol. 2011 Mar;300(3):C496-505. doi: 10.1152/ajpcell.00292.2010. Epub 2010 Dec 9. Am J Physiol Cell Physiol. 2011. PMID: 21148403 Free PMC article.
-
Transport of the photodynamic therapy agent 5-aminolevulinic acid by distinct H+-coupled nutrient carriers coexpressed in the small intestine.J Pharmacol Exp Ther. 2010 Jan;332(1):220-8. doi: 10.1124/jpet.109.159822. Epub 2009 Sep 29. J Pharmacol Exp Ther. 2010. PMID: 19789362 Free PMC article.
-
Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport.Nat Commun. 2020 Sep 22;11(1):4791. doi: 10.1038/s41467-020-18536-z. Nat Commun. 2020. PMID: 32963229 Free PMC article.
References
-
- BERLIOZ F., MAORET J.J., PARIS H., LABURTHE M., FARINOTTI R., ROZE C. alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J. Pharmacol. Exp. Ther. 2000;294:466–472. - PubMed
-
- BLOOM S.R., POLAK J.M., PEARSE A.G.E. Vasoactive intestinal peptide and watery diarrhoea syndrome. Lancet. 1973;2:14–16. - PubMed
-
- BRANT S.R., YUN C.H.C., DONOWITZ M., TSE C.M. Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am. J. Physiol. 1995;269:C198–C206. - PubMed
-
- BROWN D.R., MILLER R.J.Neurohormonal control of fluid and electrolyte transport in intestinal mucosa Handbook of Physiology 1991Besthesda, MD: American Physiological Society; 527–587.(section 6, Vol 4)eds. Field, M. & Frizzell, R.A. pp
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

