Thrombin-induced conversion of fibrinogen to fibrin results in rapid platelet trapping which is not dependent on platelet activation or GPIb
- PMID: 12598411
- PMCID: PMC1573703
- DOI: 10.1038/sj.bjp.0705095
Thrombin-induced conversion of fibrinogen to fibrin results in rapid platelet trapping which is not dependent on platelet activation or GPIb
Abstract
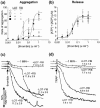
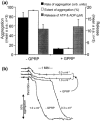
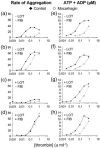
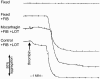
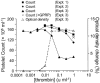
1. Activation of human platelets by thrombin is mediated by the proteolytic cleavage of two G-protein coupled protease-activated receptors, PAR-1 and PAR-4. However, thrombin also binds specifically to the platelet surface glycoprotein GPIb. It has been claimed that thrombin can induce aggregation of platelets via a novel GPIb-mediated pathway, which is independent of PAR activation and fibrinogen binding to alpha(IIb)beta(3) integrin, but dependent upon polymerizing fibrin and the generation of intracellular signals. 2. In the presence of both fibrinogen and the alpha(IIb)beta(3) receptor antagonist lotrafiban, thrombin induced a biphasic platelet aggregation response. The initial primary response was small but consistent and associated with the release of platelet granules. The delayed secondary response was more substantial and was abolished by the fibrin polymerization blocking peptide GPRP. 3. Cleavage of the extracellular portion of GPIb by mocarhagin partially inhibited thrombin-induced alpha(IIb)beta(3)-dependent aggregation and release, but had no effect on the secondary fibrin-dependent response. 4. Fixing of the platelets abolished alpha(IIb)beta(3)-dependent aggregation and release of adenine nucleotides, whereas the fibrin-dependent response remained, indicating that platelet activation and intracellular signalling are not necessary for this secondary 'aggregation'. 5. In conclusion, the secondary fibrin-dependent 'aggregation' response observed in the presence of fibrinogen and lotrafiban is a platelet trapping phenomenon dependent primarily on the conversion of soluble fibrinogen to polymerizing fibrin by thrombin.
Figures

Similar articles
-
Unique pathway of thrombin-induced platelet aggregation mediated by glycoprotein Ib.J Biol Chem. 2001 Jun 15;276(24):21173-83. doi: 10.1074/jbc.M008249200. Epub 2001 Mar 30. J Biol Chem. 2001. PMID: 11283012
-
The interaction of thrombin with blood platelets.Platelets. 2005 Nov;16(7):373-85. doi: 10.1080/09537100500123568. Platelets. 2005. PMID: 16236598 Review.
-
Thrombin binding to GPIbalpha induces platelet aggregation and fibrin clot retraction supported by resting alphaIIbbeta3 interaction with polymerized fibrin.Thromb Haemost. 2003 May;89(5):853-65. Thromb Haemost. 2003. PMID: 12719784
-
Complementary roles for fibrin(ogen), thrombospondin and vWF in mediating shear-dependent aggregation of platelets stimulated at threshold thrombin concentrations.Thromb Haemost. 2001 Aug;86(2):653-9. Thromb Haemost. 2001. PMID: 11522018
-
Platelet aggregation: involvement of thrombin and fibrin(ogen).Front Biosci. 2005 Sep 1;10:2504-17. doi: 10.2741/1715. Front Biosci. 2005. PMID: 15970513 Review.
Cited by
-
What Is the Biological and Clinical Relevance of Fibrin?Semin Thromb Hemost. 2016 Jun;42(4):333-43. doi: 10.1055/s-0036-1571342. Epub 2016 Apr 7. Semin Thromb Hemost. 2016. PMID: 27056152 Free PMC article. Review.
-
Platelet binding to polymerizing fibrin is avidity driven and requires activated αIIbβ3 but not fibrin cross-linking.Blood Adv. 2021 Oct 26;5(20):3986-4002. doi: 10.1182/bloodadvances.2021005142. Blood Adv. 2021. PMID: 34647980 Free PMC article.
-
Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage.Int J Mol Sci. 2023 Jul 25;24(15):11902. doi: 10.3390/ijms241511902. Int J Mol Sci. 2023. PMID: 37569277 Free PMC article. Review.
-
Sepsis-Associated Encephalopathy and Blood-Brain Barrier Dysfunction.Inflammation. 2021 Dec;44(6):2143-2150. doi: 10.1007/s10753-021-01501-3. Epub 2021 Jul 21. Inflammation. 2021. PMID: 34291398 Free PMC article. Review.
-
A role for adhesion and degranulation-promoting adapter protein in collagen-induced platelet activation mediated via integrin α(2) β(1).J Thromb Haemost. 2012 Feb;10(2):268-77. doi: 10.1111/j.1538-7836.2011.04567.x. J Thromb Haemost. 2012. PMID: 22103309 Free PMC article.
References
-
- ACHYUTHAN K.E., DOBSON J.V., GREENBERG C.S. Gly-Pro-Arg-Pro modifies the glutamine residues in the alpha- and gamma-chains of fibrinogen: inhibition of transglutaminase cross-linking. Biochim. Biophys. Acta. 1986;872:261–268. - PubMed
-
- ADELMAN B., GENNINGS C., STRONY J., HANNERS E. Synergistic inhibition of platelet aggregation by fibrinogen-related peptides. Circ. Res. 1990;67:941–947. - PubMed
-
- AHN H.S., ARIK L., BOYKOW G., BURNETT D.A., CAPLEN M.A., CZARNIECKI M., DOMALSKI M.S., FOSTER C., MANNA M., STAMFORD A.W., WU Y. Structure-activity relationships of pyrroloquinazolines as thrombin receptor antagonists. Bioorg. Med. Chem. Lett. 1999;9:2073–2078. - PubMed
-
- AHN H.S., FOSTER C., BOYKOW G., STAMFORD A., MANNA M., GRAZIANO M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem. Pharmacol. 2000;60:1425–1434. - PubMed
-
- BEVAN J., HEPTINSTALL S. Serotonin-induced platelet aggregation in whole blood and the effects of ketanserin and mepyramine. Thromb. Res. 1985;38:189–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

