Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost
- PMID: 12598441
- PMCID: PMC1771533
- DOI: 10.1136/bjo.87.3.297
Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost
Abstract
Aim: To investigate the effects of a non-steroidal anti-inflammatory drug (NSAID) ophthalmic solution on latanoprost induced intraocular pressure (IOP) reduction using normal volunteers.
Methods: This study was conducted as a prospective and observer masked clinical trial. 13 normal volunteers were enrolled. After measurement of basal IOP and ophthalmic examination, latanoprost ophthalmic solution was initially administered to both eyes once daily. Four weeks later, an NSAID ophthalmic solution, sodium 2-amino-3-(4-bromobenzoyl) phenylacetate sesquihydrate (refer to bromfenac sodium hydrate), was co-administered to one randomly selected eye (NSAID group) twice daily for 2 weeks. The other eye was employed as a control (non-NSAID group). After withdrawal of the NSAID ophthalmic solution, latanoprost ophthalmic solution was continuously administered for another 2 weeks and was then withdrawn. After a 4 week washout, only bromfenac sodium hydrate ophthalmic solution was administered to the eyes of the NSAID group for 2 weeks. During the study period, ophthalmic examination, including IOP measurement was performed in an observer masked fashion.
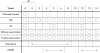
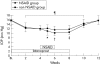
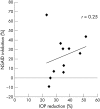
Results: Before initiation of bromfenac sodium hydrate, baseline IOPs of the non-NSAID group and the NSAID group were 15.73 (SD 1.97) mm Hg and 15.86 (2.06) mm Hg, respectively (p=0.88). Although latanoprost ophthalmic solution significantly reduced IOP in both groups, co-administration of bromfenac sodium hydrate significantly inhibited latanoprost induced IOP reduction compared with the non-NSAID group. The IOPs of the non-NSAID and NSAID groups were 10.18 (1.17) mm Hg and 11.63 (1.35) mm Hg with a 2 week co-administration, respectively (p <0.01). Withdrawal of bromfenac sodium hydrate ophthalmic solution diminished the difference between the two groups. Re-administration of bromfenac sodium ophthalmic solution only did not affect IOP.
Conclusion: These results indicate that NSAID ophthalmic solution may interfere with IOP reduction by latanoprost ophthalmic solution in normal volunteers and that we should take this into account when treating patients with glaucoma using latanoprost ophthalmic solution.
Figures
Similar articles
-
Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost in patients with primary open angle glaucoma or ocular hypertension.Br J Ophthalmol. 2006 Mar;90(3):314-7. doi: 10.1136/bjo.2005.080895. Br J Ophthalmol. 2006. PMID: 16488953 Free PMC article. Clinical Trial.
-
Effect of Travoprost and Nonsteroidal Anti-Inflammatory Drug on Diurnal Intraocular Pressure in Normal Subjects with Low-Teen Baseline Intraocular Pressure.J Ocul Pharmacol Ther. 2016 Jul-Aug;32(6):365-70. doi: 10.1089/jop.2015.0159. Epub 2016 Jun 13. J Ocul Pharmacol Ther. 2016. PMID: 27294589 Clinical Trial.
-
Effect of different dose schedules of latanoprost on intraocular pressure and pupil size in the glaucomatous Beagle.Vet Ophthalmol. 2001 Dec;4(4):283-8. doi: 10.1046/j.1463-5216.2001.00201.x. Vet Ophthalmol. 2001. PMID: 11906665 Clinical Trial.
-
Latanoprost and cystoid macular edema: is there a causal relation?Curr Opin Ophthalmol. 2000 Apr;11(2):94-100. doi: 10.1097/00055735-200004000-00005. Curr Opin Ophthalmol. 2000. PMID: 10848227 Review.
-
A case of sodium bromfenac eye drop-induced toxic epidermal necrolysis and literature review.Arch Dermatol Res. 2024 May 11;316(5):167. doi: 10.1007/s00403-024-02914-4. Arch Dermatol Res. 2024. PMID: 38734855 Review. No abstract available.
Cited by
-
Effect of a Topical Combination of Latanoprost and Pranoprofen on Intraocular Pressure and the Ocular Surface in Open-Angle Glaucoma Patients.J Ophthalmol. 2018 Dec 13;2018:7474086. doi: 10.1155/2018/7474086. eCollection 2018. J Ophthalmol. 2018. PMID: 30647962 Free PMC article.
-
Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost in patients with primary open angle glaucoma or ocular hypertension.Br J Ophthalmol. 2006 Mar;90(3):314-7. doi: 10.1136/bjo.2005.080895. Br J Ophthalmol. 2006. PMID: 16488953 Free PMC article. Clinical Trial.
-
Pathogenesis of Uveitic Glaucoma.J Curr Glaucoma Pract. 2018 Sep-Dec;12(3):125-138. doi: 10.5005/jp-journals-10028-1257. J Curr Glaucoma Pract. 2018. PMID: 31354205 Free PMC article. Review.
-
The IOP-lowering effects and mechanism of action of tafluprost in prostanoid receptor-deficient mice.Br J Ophthalmol. 2007 May;91(5):673-6. doi: 10.1136/bjo.2006.105585. Epub 2006 Nov 23. Br J Ophthalmol. 2007. PMID: 17124244 Free PMC article.
-
Can NSAIDs and prostaglandin analogues be combined?Br J Ophthalmol. 2006 Mar;90(3):259-60. doi: 10.1136/bjo.2005.083345. Br J Ophthalmol. 2006. PMID: 16488938 Free PMC article.
References
-
- Alm A. The potential of prostaglandin derivatives in glaucoma therapy. Curr Opin Ophthalmol 1993;4:44–50. - PubMed
-
- Bito LZ. Prostaglandins: a new approach to glaucoma management with a new, intriguing side effect. Surv Ophthalmol 1997;41:S1–14. - PubMed
-
- Camras CB, Alm A. Initial clinical studies with prostaglandins and their analogues. Surv Ophthalmol 1997;41:S61–8. - PubMed
-
- Nilsson SF, Samuelsson M, Bill A, et al. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2 alpha-1-isopropylester in the cynomolgus monkey. Exp Eye Res 1989;48:707–16. - PubMed
-
- Lindsey JD, Kashiwagi K, Kashiwagi F, et al. Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: implications for uveoscleral outflow. Surv Ophthalmol 1997;41:S53–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
