Mechanisms of staurosporine induced apoptosis in a human corneal endothelial cell line
- PMID: 12598452
- PMCID: PMC1771564
- DOI: 10.1136/bjo.87.3.346
Mechanisms of staurosporine induced apoptosis in a human corneal endothelial cell line
Abstract
Background: Apoptosis very probably plays a key part in endothelial cell loss during corneal storage in organ culture as well as hypothermic storage. However, the mechanisms underlying endothelial apoptosis are poorly understood. The response of a human corneal endothelial cell (HCEC) line to staurosporine, a known inducer of apoptosis, was investigated to gain insights into the intracellular modulators that participate in endothelial cell death.
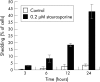
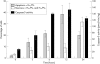
Methods: Immortalised HCECs were studied after 3, 6, 12, and 24 hours of incubation with 0.2 micro M staurosporine. Cell shedding was monitored. Hoechst 33342 fluorescent DNA staining combined with propidium iodide was used for apoptosis/necrosis quantification and morphological examination. The caspase-3 active form was assessed using western blot, proteolytic activity detection, and immunocytochemistry. The cleaved form of poly(ADP-ribose) polymerase (PARP) was assessed using immunocytochemistry and western blot. The ultrastructural features of cells were screened after 12 hours with staurosporine or vehicle.
Results: The specific apoptotic nature of staurosporine induced HCEC death was confirmed. The ultrastructural features of staurosporine treated cells were typical of apoptosis. HCEC shedding and DNA condensation increased with time. Caspase-3 activity was detected as early as 3 hours after exposure with staurosporine, peaking at 12 hours of incubation. The presence of cleaved PARP after 3 hours confirmed caspase-3 activation.
Conclusions: These data suggest strongly that HCEC cell death induced by staurosporine is apoptosis. The main consequence of HCEC apoptosis is shedding. Staurosporine induced apoptosis of endothelial cells involves activation of caspase-3, and could be a useful model to study strategies of cell death inhibition.
Figures





Similar articles
-
Lamin Cleavage: A Reliable Marker for Studying Staurosporine-Induced Apoptosis in Corneal Tissue.Invest Ophthalmol Vis Sci. 2017 Nov 1;58(13):5802-5809. doi: 10.1167/iovs.17-21830. Invest Ophthalmol Vis Sci. 2017. PMID: 29117318
-
Rapid activation of poly(ADP-ribose) polymerase contributes to Sindbis virus and staurosporine-induced apoptotic cell death.Virology. 2002 Feb 1;293(1):164-71. doi: 10.1006/viro.2001.1253. Virology. 2002. PMID: 11853409
-
Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells.Invest Ophthalmol Vis Sci. 2004 Aug;45(8):2569-76. doi: 10.1167/iovs.03-1323. Invest Ophthalmol Vis Sci. 2004. PMID: 15277479 Free PMC article.
-
Caspase-independent cell death induced by anti-CD2 or staurosporine in activated human peripheral T lymphocytes.J Immunol. 1998 Oct 1;161(7):3375-83. J Immunol. 1998. PMID: 9759854
-
Staurosporine induced poly (ADP-ribose) polymerase independent cell death in Dictyostelium discoideum.Indian J Exp Biol. 2012 Jan;50(1):80-6. Indian J Exp Biol. 2012. PMID: 22279946
Cited by
-
Opticin exerts its anti-angiogenic activity by regulating extracellular matrix adhesiveness.J Biol Chem. 2012 Aug 10;287(33):28027-36. doi: 10.1074/jbc.M111.331157. Epub 2012 Jun 5. J Biol Chem. 2012. PMID: 22669977 Free PMC article.
-
Macrophages Cytokine Spp1 Increases Growth of Prostate Intraepithelial Neoplasia to Promote Prostate Tumor Progression.Int J Mol Sci. 2022 Apr 12;23(8):4247. doi: 10.3390/ijms23084247. Int J Mol Sci. 2022. PMID: 35457063 Free PMC article.
-
Apoptotic signaling in endothelial cells with neutrophil activation.Mol Cell Biochem. 2012 Apr;363(1-2):269-80. doi: 10.1007/s11010-011-1179-5. Epub 2011 Dec 24. Mol Cell Biochem. 2012. PMID: 22198289
-
Single-walled carbon nanotubes functionalized with sodium hyaluronate enhance bone mineralization.Braz J Med Biol Res. 2016 Feb;49(2):e4888. doi: 10.1590/1414-431X20154888. Epub 2015 Dec 4. Braz J Med Biol Res. 2016. PMID: 26648087 Free PMC article.
-
Measuring the viability of crystalline lens epithelial cells by triple Hoechst-Ethidium-Calcein-AM staining.Mol Vis. 2024 Dec 31;30:478-487. eCollection 2024. Mol Vis. 2024. PMID: 39959176 Free PMC article.
References
-
- Ahmad M, Srinivasula SM, Hegde R, et al. Identification and characterization of murine caspase-14, a new member of the caspase family. Cancer Res 1998;58:5201–5. - PubMed
-
- Alnemri ES, Livingston DJ, Nicholson DW, et al. Human ICE/CED-3 protease nomenclature. Cell 1996;87:171. - PubMed
-
- Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995;376:37–43. - PubMed
-
- Kumar S. The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol 1997;29:393–6. - PubMed
-
- Tewari M, Quan LT, O’Rourke K, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 1995;81:801–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
