Intestinal absorption of mixed micellar phylloquinone (vitamin K1) is unreliable in infants with conjugated hyperbilirubinaemia: implications for oral prophylaxis of vitamin K deficiency bleeding
- PMID: 12598499
- PMCID: PMC1721510
- DOI: 10.1136/fn.88.2.f113
Intestinal absorption of mixed micellar phylloquinone (vitamin K1) is unreliable in infants with conjugated hyperbilirubinaemia: implications for oral prophylaxis of vitamin K deficiency bleeding
Abstract
Objective: To compare the pharmacokinetics and efficacy of oral versus intravenous mixed micellar vitamin K prophylaxis in infants with cholestatic liver disease, a known risk factor for vitamin K deficiency bleeding.
Design: Prospective randomised controlled study.
Setting: Paediatric Liver Unit.
Patients: Forty four infants less than 6 months of age with conjugated hyperbilirubinaemia.
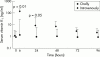
Main outcome measures: Serum concentrations of vitamin K(1) and undercarboxylated prothrombin (PIVKA-II; a sensitive functional indicator of vitamin K status) before and for up to four days after a single dose of mixed micellar K(1) 1 mg intravenously or 2 mg orally. Comparison of K(1) levels 24 hours after oral K(1) with those from 14 healthy newborns given the same dose.
Results: At admission, 18 infants (41%) had elevated levels of serum PIVKA-II and eight (18%) had low K(1) concentrations, indicative of subclinical vitamin K deficiency. Median serum K(1) concentrations were similar in the oral and intravenous groups at baseline (0.92 v 1.15 ng/ml), rising to 139 ng/ml six hours after intravenous K(1) but to only 1.4 ng/ml after oral administration. In the latter group, the low median value (0.95 ng/ml) and wide range (< 0.15-111 ng/ml) of serum K(1) compared unfavourably with the much higher levels (median 77, range 11-263 ng/ml) observed in healthy infants given the same oral dose, and suggested impaired and erratic intestinal absorption in cholestatic infants. The severity of malabsorption was such that only 4/24 (17%) achieved an incremental rise in serum K(1) > 10 ng/ml.
Conclusions: The intestinal absorption of mixed micellar K(1) is unreliable in infants with conjugated hyperbilirubinaemia. Given the strong association between cholestasis and late vitamin K deficiency bleeding, these data provide an explanation for the failure of some oral vitamin K(1) prophylaxis regimens in infants with latent cholestasis.
Figures


Comment in
-
Vitamin K--what, why, and when.Arch Dis Child Fetal Neonatal Ed. 2003 Mar;88(2):F80-3. doi: 10.1136/fn.88.2.f80. Arch Dis Child Fetal Neonatal Ed. 2003. PMID: 12598491 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
