Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells
- PMID: 12598525
- PMCID: PMC1459536
- DOI: 10.1074/jbc.M300259200
Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells
Erratum in
- J Biol Chem. 2005 Jul 8;280(27):25948
Abstract
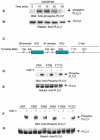
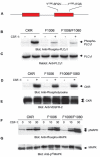
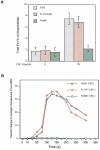
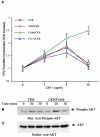
Vascular endothelial growth factor-mediated angiogenic signal transduction relay is achieved by coordinated induction of endothelial cell proliferation, migration, and differentiation. These complex cellular processes are most likely controlled by activation of both cooperative and antagonistic signals by vascular endothelial growth factor receptors (VEGFRs). Here, we investigated the contribution of tyrosine-phosphorylated residues of VEGFR-2/fetal liver kinase-1 to endothelial cell proliferation and differentiation and activation of signaling proteins. Mutation of tyrosine 1006 of VEGFR-2 to phenylalanine severely impaired the ability of this receptor to stimulate endothelial cell differentiation and tubulogenesis. Paradoxically, the mutant receptor stimulated endothelial cell proliferation far better than the wild-type receptor. Further analysis showed that tyrosine 1006 is responsible for phospholipase Cgamma1 (PLCgamma1) activation and intracellular calcium release in endothelial cells. Activation of PLCgamma1 was selectively mediated by tyrosine 1006. Mutation of tyrosines 799, 820, 949, 994, 1080, 1173, and 1221 had no measurable effect on the ability of VEGFR-2 to stimulate PLCgamma1 activation. Association of VEGFR-2 with PLCgamma1 was mainly established between tyrosine 1006 and the C-terminal SH2 domain of PLCgamma1 in vitro and in vivo. Taken together, the results indicate that phosphorylation of tyrosine 1006 is essential for VEGFR-2-mediated PLCgamma1 activation, calcium flux, and cell differentiation. More importantly, VEGFR-2-mediated endothelial cell proliferation is inversely correlated with the ability of VEGFR-2 to associate with and activate PLCgamma1.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

