Alpha 1-adrenoceptor-activated cation currents in neurones acutely isolated from rat cardiac parasympathetic ganglia
- PMID: 12598585
- PMCID: PMC2342805
- DOI: 10.1113/jphysiol.2002.033100
Alpha 1-adrenoceptor-activated cation currents in neurones acutely isolated from rat cardiac parasympathetic ganglia
Abstract
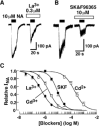
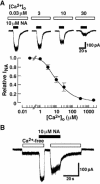
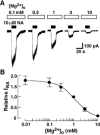
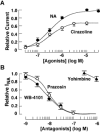
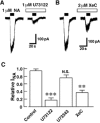
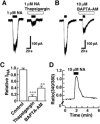
The noradrenaline (NA)-induced cation current was investigated in neurones freshly isolated from rat cardiac parasympathetic ganglia using the nystatin-perforated patch recording configuration. Under current-clamp conditions, NA depolarized the membrane, eliciting repetitive action potentials. NA evoked an inward cation current under voltage-clamp conditions at a holding potential of -60 mV. The NA-induced current was inhibited by extracellular Ca2+ or Mg2+, with a half-maximal concentration of 13 microM for Ca2+ and 1.2 mM for Mg2+. Cirazoline mimicked the NA response, and prazosin and WB-4101 inhibited the NA-induced current, suggesting the contribution of an alpha1-adrenoceptor. The NA-induced current was inhibited by U73122, a phospholipase C (PLC) inhibitor. The membrane-permeable IP3 receptor blocker xestospongin-C also blocked the NA-induced current. Furthermore, pretreatment with thapsigargin and BAPTA-AM could inhibit the NA response while KN-62, phorbol 12-myristate 13-acetate (PMA) and staurosporine had no effect. These results suggest that NA activates the extracellular Ca2+- and Mg2+-sensitive cation channels via alpha 1-adrenoceptors in neurones freshly isolated from rat cardiac parasympathetic ganglia. This activation mechanism also involves phosphoinositide breakdown, release of Ca2+ from intracellular Ca2+ stores and calmodulin. The cation channels activated by NA may play an important role in neuronal membrane depolarization in rat cardiac ganglia.
Figures


Similar articles
-
Noradrenaline-induced cation currents in isolated rat paratracheal ganglion neurons.Brain Res. 2004 Oct 8;1023(1):74-82. doi: 10.1016/j.brainres.2004.07.011. Brain Res. 2004. PMID: 15364021
-
Muscarinic receptor-mediated excitation of rat intracardiac ganglion neurons.Neuropharmacology. 2015 Aug;95:395-404. doi: 10.1016/j.neuropharm.2015.04.014. Epub 2015 Apr 29. Neuropharmacology. 2015. PMID: 25937214
-
[Excitatory effect of noradrenaline on rat airway parasympathetic ganglion neurons].Fukuoka Igaku Zasshi. 2001 Nov;92(11):377-83. Fukuoka Igaku Zasshi. 2001. PMID: 11774707 Japanese.
-
Sensing of extracellular calcium by neurones.Can J Physiol Pharmacol. 1999 Sep;77(9):715-21. Can J Physiol Pharmacol. 1999. PMID: 10566949 Review.
-
[Electrophysiology of cardiac ion channels].Nihon Rinsho. 1996 Aug;54(8):2050-5. Nihon Rinsho. 1996. PMID: 8810776 Review. Japanese.
Cited by
-
Norepinephrine-Induced Adrenergic Activation Strikingly Increased the Atrial Fibrillation Duration through β1- and α1-Adrenergic Receptor-Mediated Signaling in Mice.PLoS One. 2015 Jul 23;10(7):e0133664. doi: 10.1371/journal.pone.0133664. eCollection 2015. PLoS One. 2015. PMID: 26203906 Free PMC article.
-
Alpha-1 adrenergic input to solitary nucleus neurones: calcium oscillations, excitation and gastric reflex control.J Physiol. 2005 Jan 15;562(Pt 2):553-68. doi: 10.1113/jphysiol.2004.076919. Epub 2004 Nov 11. J Physiol. 2005. PMID: 15539398 Free PMC article.
-
Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study.Brain Res. 2011 Apr 18;1385:47-55. doi: 10.1016/j.brainres.2011.02.029. Epub 2011 Feb 18. Brain Res. 2011. PMID: 21334313 Free PMC article.
-
Expression and localization of α2A-adrenergic receptor in the rat post-natal developing cochlea.Eur J Histochem. 2023 Aug 7;67(3):3748. doi: 10.4081/ejh.2023.3748. Eur J Histochem. 2023. PMID: 37548252 Free PMC article.
-
Leptin "gates" thermogenic action of thyrotropin-releasing hormone in the hindbrain.Brain Res. 2009 Oct 27;1295:135-41. doi: 10.1016/j.brainres.2009.07.063. Epub 2009 Jul 28. Brain Res. 2009. PMID: 19643094 Free PMC article.
References
-
- Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

