Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia
- PMID: 12598608
- PMCID: PMC6742286
- DOI: 10.1523/JNEUROSCI.23-04-01198.2003
Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia
Abstract
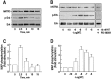
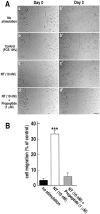
Microglia motility plays a crucial role in response to lesion or exocytotoxic damage of the cerebral tissue. We used two in vitro assays, a wound-healing model and a chemotaxis assay, to show that the neuropeptide neurotensin elicited the migration of the human microglial cell line C13NJ by a mechanism dependent on both phosphatidylinositol 3-kinase (PI 3-kinase) and mitogen-activated protein (MAP) kinase pathways. The effect of neurotensin on cell migration was blocked by the neurotensin receptor-3 propeptide, a selective ligand of this receptor. We demonstrate, by using RT-PCR, photoaffinity labeling, and Western blot analysis, that the type I neurotensin receptor-3 was the only known neurotensin receptor expressed in these microglial cells and that its activation led to the phosphorylation of both extracellular signal-regulating kinases 1/2 and Akt. Furthermore, the effect of neurotensin on cell migration was preceded by a profound modification of the F-actin cytoskeleton, particularly by the rapid formation of numerous cell filopodia. Both the motility and the filopodia appearance induced by neurotensin were totally blocked by selective inhibitors of MAP kinases or PI 3-kinase pathways. This demonstrates that the neurotensin receptor-3 is functional and mediates the migratory actions of neurotensin.
Figures




Similar articles
-
Neurotensin and the neurotensin receptor-3 in microglial cells.J Neurosci Res. 2005 Aug 1;81(3):322-6. doi: 10.1002/jnr.20477. J Neurosci Res. 2005. PMID: 15957186 Review.
-
Neurotensin receptor-3/sortilin mediates neurotensin-induced cytokine/chemokine expression in a murine microglial cell line.J Neurosci Res. 2004 Oct 1;78(1):92-9. doi: 10.1002/jnr.20231. J Neurosci Res. 2004. PMID: 15372498
-
Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells.Regul Pept. 2004 Aug 15;120(1-3):155-66. doi: 10.1016/j.regpep.2004.03.004. Regul Pept. 2004. PMID: 15177934
-
Fcgamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction.J Leukoc Biol. 2004 Jun;75(6):1147-55. doi: 10.1189/jlb.0403128. Epub 2004 Feb 24. J Leukoc Biol. 2004. PMID: 14982949
-
[Neurotensin: reception and intracellular mechanisms of signaling].Usp Fiziol Nauk. 2006 Apr-Jun;37(2):52-70. Usp Fiziol Nauk. 2006. PMID: 16758885 Review. Russian.
Cited by
-
Gender Related Changes in Gene Expression Induced by Valproic Acid in A Mouse Model of Autism and the Correction by S-adenosyl Methionine. Does It Explain the Gender Differences in Autistic Like Behavior?Int J Mol Sci. 2019 Oct 24;20(21):5278. doi: 10.3390/ijms20215278. Int J Mol Sci. 2019. PMID: 31652960 Free PMC article.
-
The effect of sortilin silencing on ovarian carcinoma cells.Avicenna J Med Biotechnol. 2014 Jul;6(3):169-77. Avicenna J Med Biotechnol. 2014. PMID: 25215181 Free PMC article.
-
Deciphering Mechanisms of Action of Sortilin/Neurotensin Receptor-3 in the Proliferation Regulation of Colorectal and Other Cancers.Int J Mol Sci. 2022 Oct 6;23(19):11888. doi: 10.3390/ijms231911888. Int J Mol Sci. 2022. PMID: 36233189 Free PMC article. Review.
-
Human pluripotent stem cell (hPSC)-derived microglia for the study of brain disorders. A comprehensive review of existing protocols.IBRO Neurosci Rep. 2024 Mar 19;16:497-508. doi: 10.1016/j.ibneur.2024.03.005. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 38655500 Free PMC article. Review.
-
The Neurobiological Underpinnings of Obsessive-Compulsive Symptoms in Psychosis, Translational Issues for Treatment-Resistant Schizophrenia.Biomolecules. 2023 Aug 5;13(8):1220. doi: 10.3390/biom13081220. Biomolecules. 2023. PMID: 37627285 Free PMC article. Review.
References
-
- Betancur C, Azzi M, Rostène W. Nonpeptide antagonists of neuropeptide receptors: tools for research and therapy. Trends Pharmacol Sci. 1997;18:372–383. - PubMed
-
- Botto JM, Chabry J, Sarret P, Vincent JP, Mazella J. Stable expression of the mouse levocabastine-sensitive neurotensin receptor in HEK 293 cell line. Binding properties, photoaffinity labeling and internalization mechanism. Biochem Biophys Res Commun. 1998;243:585–590. - PubMed
-
- Brockhaus J, Moller T, Kettenmann H. Phagocytozing ameboid microglial cells studied in a mouse corpus callosum slice preparation. Glia. 1996;16:81–90. - PubMed
-
- Chabry J, Gaudriault G, Vincent JP, Mazella J. Implication of various forms of neurotensin receptors in the mechanism of internalization of neurotensin in cerebral neurons. J Biol Chem. 1993;268:17138–17144. - PubMed
-
- Chabry J, Labbé-Jullié C, Gully D, Kitabgi P, Vincent JP, Mazella J. Stable expression of the cloned rat brain neurotensin receptor into fibroblasts: binding properties, photoaffinity labeling, transduction mechanisms, and internalization. J Neurochem. 1994;63:19–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
