Modulation of spike-mediated synaptic transmission by presynaptic background Ca2+ in leech heart interneurons
- PMID: 12598609
- PMCID: PMC6742253
- DOI: 10.1523/JNEUROSCI.23-04-01206.2003
Modulation of spike-mediated synaptic transmission by presynaptic background Ca2+ in leech heart interneurons
Abstract
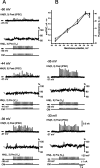
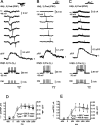
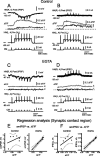
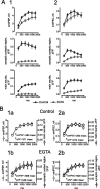
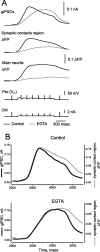
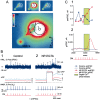
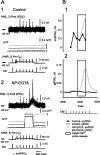
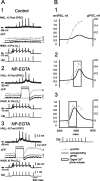
At the core of the rhythmically active leech heartbeat central pattern generator are pairs of mutually inhibitory interneurons. Synaptic transmission between these interneurons consists of spike-mediated and graded components, both of which wax and wane on a cycle-by-cycle basis. Low-threshold Ca2+ currents gate the graded component. Ca imaging experiments indicate that these low-threshold currents are widespread in the neurons and that they contribute to neuron-wide changes in internal background Ca2+ concentration (Ivanov and Calabrese, 2000). During normal rhythmic activity, background Ca2+ concentration oscillates, and thus graded synaptic transmission waxes and wanes as the neurons move from the depolarized to the inhibited phases of their activity. Here we show that in addition to gating graded transmitter release, the background Ca2+ concentration changes evoked by low-threshold Ca2+ currents modulate spike-mediated synaptic transmission. We develop stimulation paradigms to simulate the changes in baseline membrane potential that accompany rhythmic bursting. Using Ca imaging and electrophysiological measurements, we show that the strength of spike-mediated synaptic transmission follows the changes in background Ca2+ concentration that these baseline potential changes evoke and that it does not depend on previous spike activity. Moreover, we show using internal EGTA and photo-release of caged Ca2+ and caged Ca2+ chelator that changes in internal Ca2+ concentration modulate spike-mediated synaptic transmission. Thus activity-dependent changes in background Ca2+, which have been implicated in homeostatic regulation of intrinsic membrane currents and synaptic strength, may also regulate synaptic transmission in an immediate way to modulate synaptic strength cycle by cycle in rhythmically active networks.
Figures


Similar articles
-
Spike-mediated and graded inhibitory synaptic transmission between leech interneurons: evidence for shared release sites.J Neurophysiol. 2006 Jul;96(1):235-51. doi: 10.1152/jn.01094.2005. Epub 2006 Apr 26. J Neurophysiol. 2006. PMID: 16641378
-
Functional role of Ca2+ currents in graded and spike-mediated synaptic transmission between leech heart interneurons.J Neurophysiol. 1997 Apr;77(4):1779-94. doi: 10.1152/jn.1997.77.4.1779. J Neurophysiol. 1997. PMID: 9114236
-
Intracellular Ca2+ dynamics during spontaneous and evoked activity of leech heart interneurons: low-threshold Ca currents and graded synaptic transmission.J Neurosci. 2000 Jul 1;20(13):4930-43. doi: 10.1523/JNEUROSCI.20-13-04930.2000. J Neurosci. 2000. PMID: 10864951 Free PMC article.
-
Heartbeat control in the medicinal leech: a model system for understanding the origin, coordination, and modulation of rhythmic motor patterns.J Neurobiol. 1995 Jul;27(3):390-402. doi: 10.1002/neu.480270311. J Neurobiol. 1995. PMID: 7673897 Review.
-
Neural control of heartbeat in the leech and in some other invertebrates.Physiol Rev. 1979 Jan;59(1):101-36. doi: 10.1152/physrev.1979.59.1.101. Physiol Rev. 1979. PMID: 220645 Review.
Cited by
-
Repetition priming-induced changes in sensorimotor transmission.J Neurophysiol. 2016 Mar;115(3):1637-43. doi: 10.1152/jn.01082.2015. Epub 2016 Jan 13. J Neurophysiol. 2016. PMID: 26763783 Free PMC article.
-
Effect of presynaptic membrane potential on electrical vs. chemical synaptic transmission.J Neurophysiol. 2011 Aug;106(2):680-9. doi: 10.1152/jn.00340.2011. Epub 2011 May 18. J Neurophysiol. 2011. PMID: 21593394 Free PMC article.
-
Activity-dependent increases in [Ca2+]i contribute to digital-analog plasticity at a molluscan synapse.J Neurophysiol. 2017 Jun 1;117(6):2104-2112. doi: 10.1152/jn.00034.2017. Epub 2017 Mar 8. J Neurophysiol. 2017. PMID: 28275057 Free PMC article.
-
Creation and reduction of a morphologically detailed model of a leech heart interneuron.J Neurophysiol. 2006 Oct;96(4):2107-20. doi: 10.1152/jn.00026.2006. Epub 2006 Jun 7. J Neurophysiol. 2006. PMID: 16760352 Free PMC article.
-
A role for compromise: synaptic inhibition and electrical coupling interact to control phasing in the leech heartbeat CpG.Front Behav Neurosci. 2010 Jul 12;4:38. doi: 10.3389/fnbeh.2010.00038. eCollection 2010. Front Behav Neurosci. 2010. PMID: 20700387 Free PMC article.
References
-
- Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. - PubMed
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. - PubMed
-
- Augustine GJ, Adler EM, Charlton MP, Hans M, Swandulla D, Zipser K. Presynaptic calcium signals during neurotransmitter release: detection with fluorescent indicators and other calcium chelators. J Physiol (Paris) 1992;86:129–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
