Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease
- PMID: 12598611
- PMCID: PMC6742266
- DOI: 10.1523/JNEUROSCI.23-04-01228.2003
Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease
Abstract
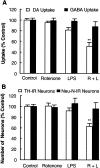
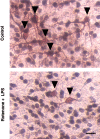
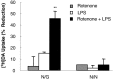
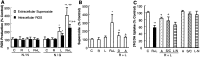
Parkinson's disease (PD) is characterized by a progressive degeneration of the nigrostriatal dopaminergic pathway resulting in movement disorders. Although its etiology remains unknown, PD may be the final outcome of interactions among multiple factors, including exposure to environmental toxins and the occurrence of inflammation in the brain. In this study, using primary mesencephalic cultures, we observed that nontoxic or minimally toxic concentrations of the pesticide rotenone (0.5 nm) and the inflammogen lipopolysaccharide (LPS) (0.5 ng/ml) synergistically induced dopaminergic neurodegeneration. The synergistic neurotoxicity of rotenone and LPS was observed when the two agents were applied either simultaneously or in tandem. Mechanistically, microglial NADPH oxidase-mediated generation of reactive oxygen species appeared to be a key contributor to the synergistic dopaminergic neurotoxicity. This conclusion was based on the following observations. First, inhibition of NADPH oxidase or scavenging of free radicals afforded significant neuroprotection. Second, rotenone and LPS synergistically stimulated the NADPH oxidase-mediated release of the superoxide free radical. Third and most importantly, rotenone and LPS failed to induce the synergistic neurotoxicity as well as the production of superoxide in cultures from NADPH oxidase-deficient animals. This is the first demonstration that low concentrations of a pesticide and an inflammogen work in synergy to induce a selective degeneration of dopaminergic neurons. Findings from this study may be highly relevant to the elucidation of the multifactorial etiology of PD and the discovery of effective therapeutic agents for the treatment of the disease.
Figures


References
-
- Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor NS-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke. 2001;32:2370–2375. - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
-
- Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704:112–116. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
