Honeycomb-like mosaic at the border of layers 1 and 2 in the cerebral cortex
- PMID: 12598625
- PMCID: PMC6742255
- DOI: 10.1523/JNEUROSCI.23-04-01372.2003
Honeycomb-like mosaic at the border of layers 1 and 2 in the cerebral cortex
Abstract
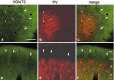
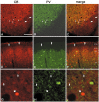
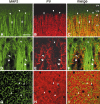
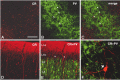
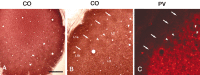
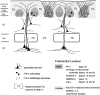
In this report, we present evidence of a small-scale modularity (<100 microm) at the border of layers 1 and 2 in neocortical areas. The modularity is best seen in tangential sections, with double-labeling immunohistochemistry to reveal overlapping or complementary relationships of different markers. The pattern is overall like a reticulum or mosaic but is described as a "honeycomb," in which the walls and hollows are composed of distinct afferent and dendritic systems. We demonstrate the main components of the honeycomb in rat visual cortex. These are as follows: (1) zinc-enriched, corticocortical terminations in the walls, and in the hollows, thalamocortical terminations (labeled by antibody against vesicular glutamate transporter 2 and by cytochrome oxidase); (2) parvalbumin-dense neuropil in the walls that partly colocalizes with elevated levels of glutamate receptors 2/3, NMDAR receptor 1, and calbindin; and (3) dendritic subpopulations preferentially situated within the walls (dendrites of layer 2 neurons) or hollows (dendrites of deeper neurons in layers 3 and 5). Because the micromodularity is restricted to layers 2 and 1b, without extending into layer 3, this may be another indication of a laminar-specific substructure at different spatial scales within cortical columns. The suggestion is that corticocortical and thalamocortical terminations constitute parallel circuits at the level of layer 2, where they are segregated in association with distinct dendritic systems. Results from parvalbumin staining show that the honeycomb mosaic is not limited to rat visual cortex but can be recognized at the layer 1-2 border in other areas and species.
Figures






Similar articles
-
Connectivity of GABAergic calretinin-immunoreactive neurons in rat primary visual cortex.Cereb Cortex. 1999 Oct-Nov;9(7):683-96. doi: 10.1093/cercor/9.7.683. Cereb Cortex. 1999. PMID: 10554991
-
Region specific micromodularity in the uppermost layers in primate cerebral cortex.Cereb Cortex. 2004 Nov;14(11):1173-84. doi: 10.1093/cercor/bhh077. Epub 2004 May 13. Cereb Cortex. 2004. PMID: 15142953
-
Zinc-rich transient vertical modules in the rat retrosplenial cortex during postnatal development.Neuroscience. 2006;138(2):523-35. doi: 10.1016/j.neuroscience.2005.11.049. Epub 2006 Jan 19. Neuroscience. 2006. PMID: 16426767
-
Calcium-binding proteins as markers for subpopulations of GABAergic neurons in monkey striate cortex.J Comp Neurol. 1990 Aug 1;298(1):1-22. doi: 10.1002/cne.902980102. J Comp Neurol. 1990. PMID: 2170466
-
Distribution of the calcium-binding proteins parvalbumin and calbindin-D28k in the sensorimotor cortex of the rat.Neuroscience. 1991;44(1):157-71. doi: 10.1016/0306-4522(91)90258-p. Neuroscience. 1991. PMID: 1770994
Cited by
-
Modularity in the Organization of Mouse Primary Visual Cortex.Neuron. 2015 Aug 5;87(3):632-43. doi: 10.1016/j.neuron.2015.07.004. Neuron. 2015. PMID: 26247867 Free PMC article.
-
Small-scale module of the rat granular retrosplenial cortex: an example of the minicolumn-like structure of the cerebral cortex.Front Neuroanat. 2012 Jan 10;5:69. doi: 10.3389/fnana.2011.00069. eCollection 2012. Front Neuroanat. 2012. PMID: 22275884 Free PMC article.
-
Dissecting cell-type-specific pathways in medial entorhinal cortical-hippocampal network for episodic memory.J Neurochem. 2023 Jul;166(2):172-188. doi: 10.1111/jnc.15850. Epub 2023 May 30. J Neurochem. 2023. PMID: 37248771 Free PMC article. Review.
-
The basic repeating modules of the cerebral cortical circuit.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(7):303-311. doi: 10.2183/pjab.95.022. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 31406055 Free PMC article. Review.
-
Vascularization of cytochrome oxidase-rich blobs in the primary visual cortex of squirrel and macaque monkeys.J Neurosci. 2011 Jan 26;31(4):1246-53. doi: 10.1523/JNEUROSCI.2765-10.2011. J Neurosci. 2011. PMID: 21273409 Free PMC article.
References
-
- Akagi T, Kaneda M, Ishii K, Hashikawa T. Differential subcellular localization of zinc in the rat retina. J Histochem Cytochem. 2001;49:87–96. - PubMed
-
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. - PubMed
-
- Bear MF. Progress in understanding NMDA-receptor-dependent synaptic plasticity in the visual cortex. J Physiol (Paris) 1996;90:223–227. - PubMed
-
- Benke D, Mertens S, Mohler H. Ubiquitous presence of GABAa receptors containing the alpha1-subunit in rat brain demonstrated by immunoprecipitation and immunohistochemistry. Mol Neuropharmacol. 1991;1:103–110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
