Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord
- PMID: 12598631
- PMCID: PMC6742279
- DOI: 10.1523/JNEUROSCI.23-04-01424.2003
Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord
Abstract
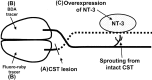
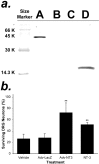
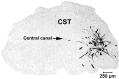
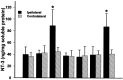
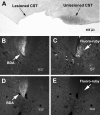
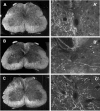
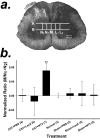
The mammalian CNS lacks the ability to effectively compensate for injury by the regeneration of damaged axons or axonal plasticity of intact axons. However, reports suggest that molecular or cellular manipulations can induce compensatory processes that could support regeneration or plasticity after trauma. We tested whether local, sustained release of the neurotrophic factor neurotrophin-3 (NT-3) would support axonal plasticity in the spinal cord distal to the site of injury in rats. The corticospinal tract (CST) was cut unilaterally at the level of the medulla. This avoided excessive inflammation, secondary cell death, vascular disruption, and the release of inhibitory molecules in the lumbar spinal cord. A replication-defective adenoviral vector (Adv) carrying the NT-3 gene (Adv.EFalpha-NT3) was delivered to the spinal motoneurons by retrograde transport through the sciatic nerve. Retrograde transport of the adenoviral vectors avoided the inflammatory response that would be associated with direct injection into the spinal cord. Transduction of spinal motoneurons with Adv.EFalpha-NT3 resulted in a significant increase in the concentration of NT-3 in the L3-L6 region of the spinal cord for up to 3 weeks. In animals with a CST lesion, this local expression of NT-3 induced growth of axons from the intact CST across the midline to the denervated side. If the CST remained intact, overexpression of NT-3 did not lead to an increase in the number of axons crossing the midline. These data demonstrate that local, sustained expression of NT-3 will support axonal plasticity of intact CST axons after trauma-induced denervation.
Figures
References
-
- Baumgartner BJ, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell-line derived neurotrophic factor. Exp Neurol. 1998a;153:102–112. - PubMed
-
- Baumgartner BJ, Shine HD. Permanent rescue of lesioned neonatal motoneurons and enhanced axonal regeneration by adenovirus-mediated expression of glial cell-line-derived neurotrophic factor. J Neurosci Res. 1998b;54:766–777. - PubMed
-
- Brösamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
