A novel functional neuron group for respiratory rhythm generation in the ventral medulla
- PMID: 12598636
- PMCID: PMC6742245
- DOI: 10.1523/JNEUROSCI.23-04-01478.2003
A novel functional neuron group for respiratory rhythm generation in the ventral medulla
Abstract
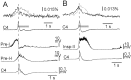
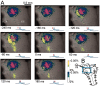
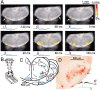
We visualized respiratory neuron activity covering the entire ventral medulla using optical recordings in a newborn rat brainstem-spinal cord preparation stained with voltage-sensitive dye. We measured optical signals from several seconds before to several seconds after the inspiratory phase using the inspiratory motor nerve discharge as the trigger signal; we averaged the optical signals of 50-150 respiratory cycles to obtain an optical image correlating particularly to inspiratory activity. The optical images we obtained from the ventral approach indicated that neuron activity first appeared during the respiratory cycle in the limited region of the rostral ventrolateral medulla (RVLM), preceding the onset of inspiratory activity by approximately 500 msec. During the inspiratory phase, plateau activity appeared in the more caudal ventrolateral medulla at the level of the most rostral roots of the XIIth nerve. Comparison with electrophysiological recordings from respiratory neurons in the RVLM suggested that the optical signals preceding the inspiratory burst reflect preinspiratory neuron activity in this area. This RVLM area was determined to be ventrolateral to the facial nucleus and close to the ventral surface. We referred to this functional neuron group as the para-facial respiratory group (pFRG). Partial, bilateral electrical lesioning of the pFRG significantly reduced the respiratory frequency, together with changes in the spatiotemporal pattern of respiratory neuron activity. Our findings suggest that the pFRG comprises a neuronal population that is involved in the primary respiratory rhythm generation in the rostrocaudally extending respiratory neuron network of the medulla.
Figures



References
-
- Arata A, Onimaru H, Homma I. Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull. 1990;24:599–604. - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem–spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. - PubMed
-
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. - PubMed
-
- Bodineau L, Cayetanot F, Frugiere A. Possible role of retrotrapezoid nucleus and parapyramidal area in the respiratory response to anoxia: an in vitro study in neonatal rat. Neurosci Lett. 2000;295:67–69. - PubMed
-
- Cohen LB, Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. In: De Weer P, Salzberg BM, editors. Optical methods in cell physiology. Wiley; New York: 1986. pp. 71–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
