Breathing: rhythmicity, plasticity, chemosensitivity
- PMID: 12598679
- PMCID: PMC2811316
- DOI: 10.1146/annurev.neuro.26.041002.131103
Breathing: rhythmicity, plasticity, chemosensitivity
Abstract
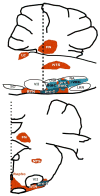
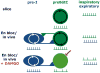
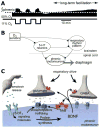
Breathing is a vital behavior that is particularly amenable to experimental investigation. We review recent progress on three problems of broad interest. (i) Where and how is respiratory rhythm generated? The preBötzinger Complex is a critical site, whereas pacemaker neurons may not be essential. The possibility that coupled oscillators are involved is considered. (ii) What are the mechanisms that underlie the plasticity necessary for adaptive changes in breathing? Serotonin-dependent long-term facilitation following intermittent hypoxia is an important example of such plasticity, and a model that can account for this adaptive behavior is discussed. (iii) Where and how are the regulated variables CO2 and pH sensed? These sensors are essential if breathing is to be appropriate for metabolism. Neurons with appropriate chemosensitivity are spread throughout the brainstem; their individual properties and collective role are just beginning to be understood.
Figures
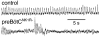
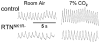
References
-
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–57. - PubMed
-
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–79. - PubMed
-
- Alheid GF, Gray PA, Habtemarkos R, Feldman JL, McCrimmon DR. Calcium binding proteins, NK1 receptors, and compartments in the ventral respiratory group of the rat. Soc Neurosci Abstr. 2001;27:1667.
-
- Aristotle . In: Aristotle VIII. Goold G, editor. Cambridge, MA: Harvard Univ. Press; 1995. p. 431.
-
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

