Rapid induction of P/C-type inactivation is the mechanism for acid-induced K+ current inhibition
- PMID: 12601085
- PMCID: PMC2217332
- DOI: 10.1085/jgp.20028760
Rapid induction of P/C-type inactivation is the mechanism for acid-induced K+ current inhibition
Abstract
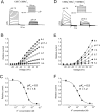
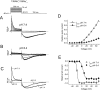
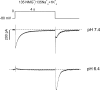
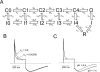
Extracellular acidification is known to decrease the conductance of many voltage-gated potassium channels. In the present study, we investigated the mechanism of H(+)(o)-induced current inhibition by taking advantage of Na(+) permeation through inactivated channels. In hKv1.5, H(+)(o) inhibited open-state Na(+) current with a similar potency to K(+) current, but had little effect on the amplitude of inactivated-state Na(+) current. In support of inactivation as the mechanism for the current reduction, Na(+) current through noninactivating hKv1.5-R487V channels was not affected by [H(+)(o)]. At pH 6.4, channels were maximally inactivated as soon as sufficient time was given to allow activation, which suggested two possibilities for the mechanism of action of H(+)(o). These were that inactivation of channels in early closed states occurred while hyperpolarized during exposure to acid pH (closed-state inactivation) and/or inactivation from the open state was greatly accelerated at low pH. The absence of outward Na(+) currents but the maintained presence of slow Na(+) tail currents, combined with changes in the Na(+) tail current time course at pH 6.4, led us to favor the hypothesis that a reduction in the activation energy for the inactivation transition from the open state underlies the inhibition of hKv1.5 Na(+) current at low pH.
Figures





Similar articles
-
Regulation of transient Na+ conductance by intra- and extracellular K+ in the human delayed rectifier K+ channel Kv1.5.J Physiol. 2000 Mar 15;523 Pt 3(Pt 3):575-91. doi: 10.1111/j.1469-7793.2000.00575.x. J Physiol. 2000. PMID: 10718739 Free PMC article.
-
Constitutive inactivation of the hKv1.5 mutant channel, H463G, in K+-free solutions at physiological pH.Cell Biochem Biophys. 2005;43(2):221-30. doi: 10.1385/CBB:43:2:221. Cell Biochem Biophys. 2005. PMID: 16049347
-
A high-Na(+) conduction state during recovery from inactivation in the K(+) channel Kv1.5.Biophys J. 2000 Nov;79(5):2416-33. doi: 10.1016/S0006-3495(00)76486-1. Biophys J. 2000. PMID: 11053120 Free PMC article.
-
Synergistic inhibition of the maximum conductance of Kv1.5 channels by extracellular K+ reduction and acidification.Cell Biochem Biophys. 2005;43(2):231-42. doi: 10.1385/CBB:43:2:231. Cell Biochem Biophys. 2005. PMID: 16049348 Review.
-
Closed-state inactivation induced in K(V)1 channels by extracellular acidification.Channels (Austin). 2008 Mar-Apr;2(2):139-42. doi: 10.4161/chan.2.2.6231. Epub 2008 Mar 6. Channels (Austin). 2008. PMID: 18849651 Review.
Cited by
-
Mechanisms underlying modulation of neuronal KCNQ2/KCNQ3 potassium channels by extracellular protons.J Gen Physiol. 2003 Dec;122(6):775-93. doi: 10.1085/jgp.200308897. J Gen Physiol. 2003. PMID: 14638935 Free PMC article.
-
Na+ permeation and block of hERG potassium channels.J Gen Physiol. 2006 Jul;128(1):55-71. doi: 10.1085/jgp.200609500. Epub 2006 Jun 12. J Gen Physiol. 2006. PMID: 16769794 Free PMC article.
-
BK channel inhibition by strong extracellular acidification.Elife. 2018 Jul 2;7:e38060. doi: 10.7554/eLife.38060. Elife. 2018. PMID: 29963986 Free PMC article.
-
Cell Settling, Migration, and Stochastic Cancer Gene Expression Suggest Potassium Membrane Flux May Initiate pH Reversal.Biomolecules. 2025 Aug 16;15(8):1177. doi: 10.3390/biom15081177. Biomolecules. 2025. PMID: 40867621 Free PMC article.
-
The external K+ concentration and mutations in the outer pore mouth affect the inhibition of kv1.5 current by Ni2+.Biophys J. 2004 Apr;86(4):2238-50. doi: 10.1016/S0006-3495(04)74282-4. Biophys J. 2004. PMID: 15041663 Free PMC article.
References
-
- Claydon, T.W., M.R. Boyett, A. Sivaprasadarao, K. Ishii, J.M. Owen, H.A. O'Beirne, R. Leach, K. Komukai, and C.H. Orchard. 2000. Inhibition of the K+ channel Kv1.4 by acidosis: protonation of an extracellular histidine slows the recovery from N-type inactivation. J. Physiol. 526:253–264. - PMC - PubMed
-
- Claydon, T.W., M.R. Boyett, A. Sivaprasadarao, and C.H. Orchard. 2002. Two pore residues mediate acidosis-induced enhancement of C-type inactivation of the Kv1.4 K+ channel. Am. J. Physiol. Cell Physiol. 283:C1114–C1121. - PubMed
-
- Ishii, K., K. Nunoki, T. Yamagishi, H. Okada, and N. Taira. 2001. Differential sensitivity of Kv1.4, Kv1.2, and their tandem channel to acidic pH: involvement of a histidine residue in high sensitivity to acidic pH. J. Pharmacol. Exp. Ther. 296:405–411. - PubMed

