On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL
- PMID: 12601086
- PMCID: PMC2217331
- DOI: 10.1085/jgp.20028768
On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL
Abstract
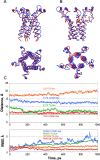
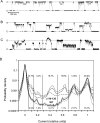
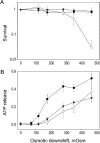
COOH-terminal (S3) domains are conserved within the MscL family of bacterial mechanosensitive channels, but their function remains unclear. The X-ray structure of MscL from Mycobacterium tuberculosis (TbMscL) revealed cytoplasmic domains forming a pentameric bundle (Chang, G., R.H. Spencer, A.T. Lee, M.T. Barclay, and D.C. Rees. 1998. SCIENCE: 282:2220-2226). The helices, however, have an unusual orientation in which hydrophobic sidechains face outside while charged residues face inside, possibly due to specific crystallization conditions. Based on the structure of pentameric cartilage protein, we modeled the COOH-terminal region of E. coli MscL to better satisfy the hydrophobicity criteria, with sidechains of conserved aliphatic residues all inside the bundle. Molecular dynamic simulations predicted higher stability for this conformation compared with one modeled after the crystal structure of TbMscL, and suggested distances for disulfide trapping experiments. The single cysteine mutants L121C and I125C formed dimers under ambient conditions and more so in the presence of an oxidant. The double-cysteine mutants, L121C/L122C and L128C/L129C, often cross-link into tetrameric and pentameric structures, consistent with the new model. Patch-clamp examination of these double mutants under moderately oxidizing or reducing conditions indicated that the bundle cross-linking neither prevents the channel from opening nor changes thermodynamic parameters of gating. Destabilization of the bundle by replacing conservative leucines with small polar residues, or complete removal of COOH-terminal domain (Delta110-136 mutation), increased the occupancy of subconducting states but did not change gating parameters substantially. The Delta110-136 truncation mutant was functional in in vivo osmotic shock assays; however, the amount of ATP released into the shock medium was considerably larger than in controls. The data strongly suggest that in contrast to previous gating models (Sukharev, S., M. Betanzos, C.S. Chiang, and H.R. Guy. 2001a. NATURE: 409:720-724.), S3 domains are stably associated in both closed and open conformations. The bundle-like assembly of cytoplasmic helices provides stability to the open conformation, and may function as a size-exclusion filter at the cytoplasmic entrance to the MscL pore, preventing loss of essential metabolites.
Figures







References
-
- Ajouz, B., C. Berrier, A. Garrigues, M. Besnard, and A. Ghazi. 1998. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J. Biol. Chem. 273:26670–26674. - PubMed
-
- Arkin, I.T., S.I. Sukharev, P. Blount, C. Kung, and A.T. Brunger. 1998. Helicity, membrane incorporation, orientation and thermal stability of the large conductance mechanosensitive ion channel from E. coli. Biochim. Biophys. Acta. 1369:131–140. - PubMed
-
- Betanzos, M., C.-S. Chiang, H.R. Guy, and S. Sukharev. 2002. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 9:704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

