Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice
- PMID: 12601154
- PMCID: PMC151396
- DOI: 10.1073/pnas.0538056100
Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice
Abstract
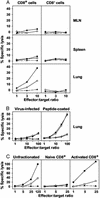
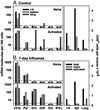
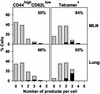
Influenza virus infection activates cytolytic T lymphocytes (CTL) that contribute to viral clearance by releasing perforin and granzymes from cytoplasmic granules. Virus-specific, perforin-dependent CD8(+) CTL were detected in freshly isolated cells from the mouse lung parenchyma but not from the mediastinal lymph nodes (MLN), where they are primed, or from the spleen during primary influenza virus infection. To determine whether this difference was due to the low frequency or incomplete maturation of effector CTL in MLN, we measured expression of perforin, granzymes A, B, and C, and IFN-gamma mRNAs in CD8(+) populations and single cells immediately after isolation from virus-infected mice. Quantitative PCR revealed significant expression of perforin, granzyme A, granzyme B, and IFN-gamma in activated CD8(+) cells from MLN, spleen, and lung parenchyma. Granzyme C expression was not detected. Individual activated or nucleoprotein peptide/class I tetramer-binding CD8(+) cells from the three tissues expressed diverse combinations of perforin, granzyme, and IFN-gamma mRNAs. Although cells from lung expressed granzymes A and B at higher frequency, each of the tissues contained cells that coexpressed perforin with granzymes A and/or B. The main difference between MLN and lung was the elevated frequency of activated CD8(+) T cells in the lung, rather than their perforin/granzyme expression profile. The data suggest that some CTL mature into perforin/granzyme-expressing effector cells in MLN but reach detectable frequencies only when they accumulate in the infected lung.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

