Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production
- PMID: 12603602
- PMCID: PMC1782892
- DOI: 10.1046/j.1365-2567.2003.01609.x
Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production
Abstract
We showed in a previous study that the intranasal (i.n) delivery of bacille Calmette-Guérin (BCG) to BP2 mice (H-2q) inhibits eosinophilia and bronchial hyperreactivity in a mouse model of asthma. The present work has been performed to characterize the leucocyte lineages recruited to the lungs of mice after i.n. delivery of BCG and potentially involved in the polarization of T lymphocytes. The different antigen-presenting cells (APC) recruited to bronchoalveolar lavage (BAL) and to lung tissue of mice shortly after the delivery of BCG were analysed in parallel as well as their capacity to drive the immune response towards a T helper type 1 cytokine production. Alveolar macrophages (AM) from the BAL were CD11c+, F4/80+ and CD11b-, and in the lung tissue two major populations of potential APC were detected: one CD11c-, F4/80+, CD11b+ and I-Aq- was identified as interstitial macrophages (IM) and a second expressing CD11c+ and I-Aq+ antigens, negative for CD11b and F4/80 markers as leucocytic dendritic cells (DC). Freshly isolated DC up-regulated CD11b and CD40 antigens after overnight culture, but remained negative for CD8alpha antigen, suggesting a myeloid origin. Lung DC which produced high amount of interleukin (IL)-12 were potent inducers of naive CD4+ T lymphocyte priming, as assessed by interferon-gamma (IFN-gamma) production by these naive CD4+ T cells. Lung explants recovered long term after BCG delivery produced sustained levels of IFN-gamma. Our results suggest that AM and particularly DC by secreting IL-12 shortly after BCG delivery induce the long-term persistence of IFN-gamma-secreting T cells percolating in BCG-loaded lung tissue.
Figures
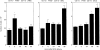


Similar articles
-
Extended freeze-dried Mycobacterium bovis Bacillus Calmette-Guérin induces the release of interleukin-12 but not tumour necrosis factor-alpha by alveolar macrophages, both in vitro and in vivo.Clin Exp Allergy. 2003 Mar;33(3):386-93. doi: 10.1046/j.1365-2222.2003.01612.x. Clin Exp Allergy. 2003. PMID: 12614454
-
Complement C5a anaphylatoxin is an innate determinant of dendritic cell-induced Th1 immunity to Mycobacterium bovis BCG infection in mice.J Leukoc Biol. 2007 Oct;82(4):956-67. doi: 10.1189/jlb.0206119. Epub 2007 Aug 3. J Leukoc Biol. 2007. PMID: 17675563
-
Pulmonary immune responses during primary mycobacterium bovis- Calmette-Guerin bacillus infection in C57Bl/6 mice.Am J Respir Cell Mol Biol. 2000 Mar;22(3):333-43. doi: 10.1165/ajrcmb.22.3.3776. Am J Respir Cell Mol Biol. 2000. PMID: 10696070
-
Development of an asthma vaccine: research into BCG.Drugs. 2000 Jun;59(6):1217-21. doi: 10.2165/00003495-200059060-00002. Drugs. 2000. PMID: 10882158 Review.
-
Pulmonary dendritic cells: sentinels of lung-associated lymphoid tissues.Am J Respir Cell Mol Biol. 1991 Mar;4(3):204-5. doi: 10.1165/ajrcmb/4.3.204. Am J Respir Cell Mol Biol. 1991. PMID: 1900423 Review. No abstract available.
Cited by
-
Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis.Clin Dev Immunol. 2012;2012:628293. doi: 10.1155/2012/628293. Epub 2012 Apr 1. Clin Dev Immunol. 2012. PMID: 22545059 Free PMC article. Review.
-
The antiasthma effect of neonatal BCG vaccination does not depend on the Th17/Th1 but IL-17/IFN-γ balance in a BALB/c mouse asthma model.J Clin Immunol. 2011 Jun;31(3):419-29. doi: 10.1007/s10875-010-9503-5. Epub 2011 Feb 22. J Clin Immunol. 2011. PMID: 21340706
-
Two Distinct Subsets Are Identified from the Peritoneal Myeloid Mononuclear Cells Expressing both CD11c and CD115.Immune Netw. 2019 May 22;19(3):e15. doi: 10.4110/in.2019.19.e15. eCollection 2019 Jun. Immune Netw. 2019. PMID: 31281712 Free PMC article.
-
Respiratory dendritic cells: mediators of tolerance and immunity.Immunol Res. 2007;39(1-3):128-45. doi: 10.1007/s12026-007-0077-0. Immunol Res. 2007. PMID: 17917061 Review.
-
The glycosylated Rv1860 protein of Mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG.PLoS Pathog. 2014 Jun 12;10(6):e1004176. doi: 10.1371/journal.ppat.1004176. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945624 Free PMC article.
References
-
- Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar lavage T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Kline JN, Hunninghake GW. T-lymphocyte dysregulation in asthma. Proc Soc Exp Biol Med. 1994;207:243–53. - PubMed
-
- Krug N, Frew AJ. The Th2 cell in asthma: initial expectations yet to be realized. Clin Exp Allergy. 1997;27:142–50. - PubMed
-
- Gajewski T, Fitch F. Anti-proliferative effect of IFN-gamma in immune regulation. Part I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–52. - PubMed
-
- Lack G, Bradey KL, Hamelmann E, Renz H, Loader J, Leung OJ, Larsen G, Gelfand GW. Nebulized IFN-γ inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

