An abnormal but functionally active complement component C9 protein found in an Irish family with subtotal C9 deficiency
- PMID: 12603605
- PMCID: PMC1782909
- DOI: 10.1046/j.1365-2567.2003.01587.x
An abnormal but functionally active complement component C9 protein found in an Irish family with subtotal C9 deficiency
Abstract
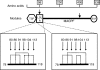
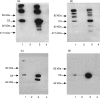
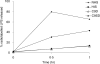
Two independently segregating C9 genetic defects have previously been reported in two siblings in an Irish family with subtotal C9 deficiency. One defect would lead to an abnormal C9 protein, with replacement of a cysteine by a glycine (C98G). The second defect is a premature stop codon at amino acid 406 which would lead to a truncated C9. However, at least one of two abnormal proteins was present in the circulation of the proband at 0.2% of normal C9 concentration. In this study, the abnormal protein was shown to have a molecular weight approximately equal to that of normal C9, and to carry the binding site for monoclonal antibody (mAb) Mc42 which is known to react with an epitope at amino acid positions 412-426, distal to 406. Therefore, the subtotal C9 protein carries the C98G defect. The protein was incorporated into the terminal complement complex, and was active in haemolytic, bactericidal and lipopolysaccharide release assays. A quantitative haemolytic assay indicated even slightly greater haemolytic efficiency than normal C9. Epitope mapping with six antihuman C9 mAbs showed the abnormal protein to react to these antibodies in the same way as normal C9. However, none of these mAbs have epitopes within the lipoprotein receptor A module, where the C98G defect is located. The role of this region in C9 functionality is still unclear. In conclusion, we have shown that the lack of a cysteine led to the production of a protein present in the circulation at very much reduced levels, but which was fully functionally active.
Figures

Similar articles
-
Several epitopes on native human complement C9 are involved in interaction with the C5b-8 complex and other C9 molecules.Eur J Immunol. 1990 Mar;20(3):623-8. doi: 10.1002/eji.1830200324. Eur J Immunol. 1990. PMID: 1690659
-
Inherited deficiency of the ninth component of complement in man.J Immunol. 1980 Nov;125(5):2252-7. J Immunol. 1980. PMID: 7430628
-
Inhibition of terminal complement complex formation and cell lysis by monoclonal antibodies.Complement Inflamm. 1991;8(5-6):328-40. doi: 10.1159/000463204. Complement Inflamm. 1991. PMID: 1724954
-
Recurrent meningitis in a patient with congenital deficiency of the C9 component of complement. First case of C9 deficiency in Europe.Arch Intern Med. 1990 Nov;150(11):2395-9. Arch Intern Med. 1990. PMID: 2241452 Review.
-
[C9 deficiency].Ryoikibetsu Shokogun Shirizu. 2000;(32):212-4. Ryoikibetsu Shokogun Shirizu. 2000. PMID: 11212695 Review. Japanese. No abstract available.
Cited by
-
Deficiencies of the complement MAC II gene cluster (C6, C7, C9): is subtotal C6 deficiency of particular evolutionary benefit?Clin Exp Immunol. 2003 Aug;133(2):156-9. doi: 10.1046/j.1365-2249.2003.02230.x. Clin Exp Immunol. 2003. PMID: 12869019 Free PMC article. Review. No abstract available.
-
Surface antibody changes protein corona both in human and mouse serum but not final opsonization and elimination of targeted polymeric nanoparticles.J Nanobiotechnology. 2023 Oct 14;21(1):376. doi: 10.1186/s12951-023-02134-4. J Nanobiotechnology. 2023. PMID: 37838659 Free PMC article.
-
Interference of the Zika Virus E-Protein With the Membrane Attack Complex of the Complement System.Front Immunol. 2020 Oct 28;11:569549. doi: 10.3389/fimmu.2020.569549. eCollection 2020. Front Immunol. 2020. PMID: 33193347 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

