Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis
- PMID: 12603729
- PMCID: PMC2562637
- DOI: 10.1046/j.1365-2958.2003.03366.x
Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis
Abstract
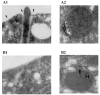
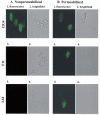
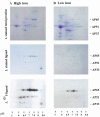
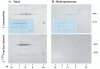
The proteins AP65, AP51, AP33 and AP23 synthesized by Trichomonas vaginalis organisms in high iron play a role in adherence. Multigene families encode enzymes of the hydrogenosome organelles, which have identity to adhesins. This fact raises questions regarding the compartmentalization of the proteins outside the organelle and about the interactions of adhesins with host cells. Data here demonstrate the presence of the proteins outside the organelle under high-iron conditions. Fluorescence and immuno-cytochemical experiments show that high-iron-grown organisms coexpressed adhesins on the surface and intracellularly in contrast with low-iron parasites. Furthermore, the AP65 epitopes seen by rabbit anti-AP65 serum that blocks adherence and detects surface proteins were identified, and a mAb reacting to those epitopes recognized the trichomonal surface. Two-dimensional electrophoresis and immunoblot of adhesins from surface-labelled parasites provided evidence that all members of the multigene family were co-ordinately expressed and placed on the trichomonal surface. Similar two-dimensional analysis of proteins from purified hydrogenosomes obtained from iodinated trichomonads confirmed the specific surface labelling of proteins. Contact of trichomonads with vaginal epithelial cells increased the amount of surface-expressed adhesins. Moreover, we found a direct relationship between the levels of adherence and amount of adhesins bound to immortalized vaginal and ureter epithelial cells, further reinforcing specific associations. Finally, trichomonads of MR100, a drug-resistant isolate absent in hydrogenosome proteins and adhesins, were non-adherent. Overall, the results confirm an important role for iron and contact in the surface expression of adhesins of T. vaginalis organisms.
Figures




References
-
- Addis MF, Rappelli P, Cappuccinelli P, Fiori PL. Extracellular release by Trichomonas vaginalis of a NADP-dependent malic enzyme involved in pathogenicity. Microbial Pathogen. 1997;23:55–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

