Senescence of the temporal impulse response to a luminous pulse
- PMID: 12604098
- PMCID: PMC2774211
- DOI: 10.1016/s0042-6989(03)00009-9
Senescence of the temporal impulse response to a luminous pulse
Abstract
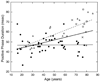
An impulse response function (IRF) to a luminous pulse was derived for 70 normal observers ranging in age from 16 to 86 years. Thresholds were measured for two pulses separated by interstimulus intervals from 6.7 to 180 ms. The pulses had a spatial Gaussian shape (+/-1SD=2.3 degrees diam) and were presented as increments on a 10 cd/m(2) background, having the same chromaticity as the pulse. A spatial 4-alternative forced-choice method was combined with a staircase procedure. Retinal illuminance was equated individually by heterochromatic flicker photometry and control of pupil area. Each IRF was measured four times, in separate sessions, for each observer. IRFs calculated from the threshold data revealed significant age-related changes in the response amplitude of both excitatory and inhibitory phases. In general, there were no significant changes in the time to the first peak or in the first zero crossing. For 12 of 20 observers over 60 years of age, however, the amplitude of the second (inhibitory) phase was reduced relative to the excitatory phase so their IRFs were quite slow and long. Control conditions with three pseudophakic observers and two normal observers with induced blur demonstrated that age-related changes in the IRF under these conditions cannot be ascribed to optical factors. The data suggest that the human visual system generally maintains a stable speed of response to a flash until at least about 80 years of age, even while there are senescent reductions in response signal amplitude.
Figures









References
-
- Benardete EA. Ph.D. dissertation. New York: The Rockefeller University; 1994. Functional dynamics of primate retinal ganglion cells.
-
- Bowen RW. Two pulses seen as three flashes: A superposition analysis. Vision Research. 1989;29:409–417. - PubMed
-
- Burr DC, Morrone MC. Impulse-response functions for chromatic and achromatic stimuli. Journal of the Optical Society of America A. 1993;10:1706–1713.
-
- Burr DC, Morrone MC. Temporal impulse response functions for luminance and colour during saccades. Vision Research. 1996;36:2069–2978. - PubMed
-
- Charman WN. Effect of refractive error in visual tests with sinusoidal gratings. British Journal of Optometry. 1979;33:10–20. - PubMed

