New anti-human immunodeficiency virus type 1 6-aminoquinolones: mechanism of action
- PMID: 12604517
- PMCID: PMC149318
- DOI: 10.1128/AAC.47.3.889-896.2003
New anti-human immunodeficiency virus type 1 6-aminoquinolones: mechanism of action
Abstract
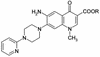
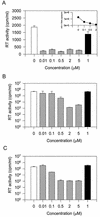
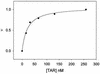
A 6-aminoquinolone derivative, WM5, which bears a methyl substituent at the N-1 position and a 4-(2-pyridyl)-1-piperazine moiety at position 7 of the bicyclic quinolone ring system, was previously shown to exhibit potent activity against replication of human immunodeficiency virus type 1 (HIV-1) in de novo-infected human lymphoblastoid cells (V. Cecchetti et al., J. Med. Chem. 43:3799-3802, 2000). In this report, we further investigated WM5's mechanism of antiviral activity. WM5 inhibited HIV-1 replication in acutely infected cells as well as in chronically infected cells. The 50% inhibitory concentrations were 0.60 +/- 0.06 and 0.85 +/- 0.05 micro M, respectively. When the effects of WM5 on different steps of the virus life cycle were analyzed, the reverse transcriptase activity and the integrase and protease activities were not impaired. By using a transient trans-complementation assay to examine the activity of WM5 on the replicative potential of HIV-1 in a single round of infection, a sustained inhibition of Tat-mediated long terminal repeat (LTR)-driven transcription (>80% of controls) was obtained in the presence of 5 micro M WM5. Interestingly, the aminoquinolone was found to efficiently complex TAR RNA, with a dissociation constant in the nanomolar range (19 +/- 0.6 nM). These data indicate that WM5 is a promising lead compound for the development of a new class of HIV-1 transcription inhibitors characterized by recognition of viral RNA target(s).
Figures


References
-
- Aboul-ela, F., J. Karn, and G. Varani. 1995. The structure of the human immunodeficiency virus type 1 TAR RNA reveals principles of RNA recognition by Tat protein. J. Mol. Biol. 253:313-332. - PubMed
-
- Antonello, C., E. Uriarte, M. Palumbo, S. Valisena, C. Parolin, and G. Palù. 1993. Synthesis and biological activity of new quinolone derivatives. Eur. J. Med. Chem. 28:291-296.
-
- Artico, M., R. Di Santo, R. Costi, E. Novellino, G. Greco, S. Massa, E. Tramontano, M. E. Marongiu, A. De Montis, and P. La Colla. 1998. Geometrically and conformationally restrained cinnamoyl compounds as inhibitors of HIV-1 integrase: synthesis, biological evaluation, and molecular modeling. J. Med. Chem. 41:3948-3960. - PubMed
-
- Baba, M., M. Okamoto, M. Kawamura, M. Makino, M. Higashida, T. Takashi, Y. Kimura, T. Ikeuchi, T. Tetsuka, and T. Okamoto. 1998. Inhibition of human immunodeficiency virus type 1 replication and cytokine production by fluoroquinoline derivatives. Mol. Pharmacol. 53:1097-1103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

