In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model
- PMID: 12604524
- PMCID: PMC149320
- DOI: 10.1128/AAC.47.3.932-940.2003
In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model
Abstract
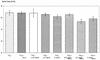
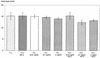
Porphyromonas gingivalis is one of the major causative organisms of periodontitis and has been shown to be susceptible to toluidine blue-mediated photosensitization in vitro. The aims of the present study were to determine whether this technique could be used to kill the organism in the oral cavities of rats and whether this would result in a reduction in the alveolar bone loss characteristic of periodontitis. The maxillary molars of rats were inoculated with P. gingivalis and exposed to up to 48 J of 630-nm laser light in the presence of toluidine blue. The number of surviving bacteria was then determined, and the periodontal structures were examined for evidence of any damage. When toluidine blue was used together with laser light there was a significant reduction in the number of viable P. gingivalis organisms. No viable bacteria could be detected when 1 mg of toluidine blue per ml was used in conjunction with all light doses used. On histological examination, no adverse effect of photosensitization on the adjacent tissues was observed. In a further group of animals, after time was allowed for the disease to develop in controls, the rats were killed and the level of maxillary molar alveolar bone was assessed. The bone loss in the animals treated with light and toluidine blue was found to be significantly less than that in the control groups. The results of this study show that toluidine blue-mediated lethal photosensitization of P. gingivalis is possible in vivo and that this results in decreased bone loss. These findings suggest that photodynamic therapy may be useful as an alternative approach for the antimicrobial treatment of periodontitis.
Figures
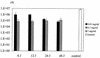
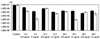



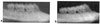
Similar articles
-
Exposure of Porphyromonas gingivalis to red light in the presence of the light-activated antimicrobial agent toluidine blue decreases membrane fluidity.Curr Microbiol. 2002 Aug;45(2):118-22. doi: 10.1007/s00284-001-0095-4. Curr Microbiol. 2002. PMID: 12070690
-
A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitization.Photochem Photobiol. 1998 Sep;68(3):370-6. Photochem Photobiol. 1998. PMID: 9747591
-
Effects of toluidine blue-mediated photodynamic therapy on periopathogens and periodontal biofilm: in vitro evaluation.Int J Immunopathol Pharmacol. 2010 Oct-Dec;23(4):1125-32. doi: 10.1177/039463201002300416. Int J Immunopathol Pharmacol. 2010. PMID: 21244761
-
Antimicrobial effect of photodynamic therapy using high-power blue light-emitting diode and red-dye agent on Porphyromonas gingivalis.J Periodontal Res. 2013 Dec;48(6):696-705. doi: 10.1111/jre.12055. Epub 2013 Feb 27. J Periodontal Res. 2013. PMID: 23441868
-
Photoinactivation and Photoablation of Porphyromonas gingivalis.Pathogens. 2023 Sep 14;12(9):1160. doi: 10.3390/pathogens12091160. Pathogens. 2023. PMID: 37764967 Free PMC article. Review.
Cited by
-
Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques.Lasers Med Sci. 2008 Jan;23(1):49-54. doi: 10.1007/s10103-007-0454-x. Epub 2007 Mar 15. Lasers Med Sci. 2008. PMID: 17361390
-
Photoexcited Toluidine Blue Inhibits Tau Aggregation in Alzheimer's Disease.ACS Omega. 2019 Oct 29;4(20):18793-18802. doi: 10.1021/acsomega.9b02792. eCollection 2019 Nov 12. ACS Omega. 2019. PMID: 31737841 Free PMC article.
-
Laser phototherapy in the treatment of periodontal disease. A review.Lasers Med Sci. 2010 Nov;25(6):781-92. doi: 10.1007/s10103-010-0812-y. Epub 2010 Jul 17. Lasers Med Sci. 2010. PMID: 20640471 Review.
-
Phototherapy and optical waveguides for the treatment of infection.Adv Drug Deliv Rev. 2021 Dec;179:114036. doi: 10.1016/j.addr.2021.114036. Epub 2021 Nov 3. Adv Drug Deliv Rev. 2021. PMID: 34740763 Free PMC article. Review.
-
Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up.Lasers Med Sci. 2012 Jul;27(4):687-93. doi: 10.1007/s10103-011-0942-x. Epub 2011 Jun 18. Lasers Med Sci. 2012. PMID: 21687979 Clinical Trial.
References
-
- Bagchi, B., and B. Sreeradha. 1989. Role of dye molecules remaining outside the cell during photodynamic inactivation of Escherichia coli in the presence of acriflavine. Photochem. Photobiol. 29:403-405. - PubMed
-
- Berthiaume, F. S., R. Reiken, M. Toner, R. G. Tompkins, and M. L. Yarmush. 1994. Antibody-targeted photolysis of bacteria in vivo. Bio/Technology 12:703-705. - PubMed
-
- Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1997. Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem. Photobiol. 65:1026-1031. - PubMed
-
- Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1998. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitisation. Photochem. Photobiol. 68:370-376. - PubMed
-
- Chaves, E. S., M. K. Jeffcoat, C. C. Ryerson, and B. Snyder. 2000. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J. Clin. Periodontol. 27:897-903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

