Comparing pharmacokinetics of amoxicillin given twice or three times per day to children older than 3 months with pneumonia
- PMID: 12604533
- PMCID: PMC149282
- DOI: 10.1128/AAC.47.3.997-1001.2003
Comparing pharmacokinetics of amoxicillin given twice or three times per day to children older than 3 months with pneumonia
Abstract
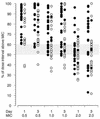
For children with ambulatory pneumonia, the World Health Organization (WHO) recommends oral amoxicillin (15 mg/kg of body weight/dose) thrice daily (t.i.d.) or oral cotrimoxazole (4 mg of trimethoprim/kg/dose) twice daily (b.i.d.). The more frequent amoxicillin dosing may lead to compliance problems. To compare the pharmacokinetics and levels of amoxicillin in plasma in the current WHO acute respiratory infection recommendations with the 25-mg/kg/dose b.i.d. regimen, we performed a two-group parallel study of 66 children ages 3 to 59 months with pneumonia. Amoxicillin was given orally at 25 mg/kg/dose b.i.d. or 15 mg/kg/dose t.i.d. Amoxicillin concentrations were determined by high-performance liquid chromatography after the first dose on days 1 and 3. After the first dose on day 1, the mean area under the concentration-time curve (AUC) for amoxicillin after the 25-mg/kg dose was 54.7 versus 24.9 micro g. h/ml after the 15-mg/kg dose. After the first dose on day 3, the mean AUC was 44.1 versus 28.5 micro g. h/ml. All but two children had plasma amoxicillin concentrations above 0.5 micro g/ml for >50% of the dose interval on both days. Six children on day 1 and five children on day 3 had concentrations above 1.0 micro g/ml for <50% of the dose interval. On day 1, 16 of 27 children in the b.i.d. group and 11 of 26 children in the t.i.d. group had concentrations that were above 2.0 micro g/ml for <50% of the dose interval, and on day 3, 18 of 31 children in the b.i.d. group and 8 of 31 children in the t.i.d. group had concentrations that were above 2.0 micro g/ml for <50% of the dose interval. Amoxicillin b.i.d. is a feasible alternative for t.i.d. dosing. To lengthen the time above the MIC at higher concentration levels, a 30- to 40-mg/kg/dose b.i.d. should be considered instead of the 25 mg/kg/dose used in this study.
Figures

References
-
- Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. - PubMed
-
- Chretien, J., W. Holland, P. Macklem, P. Murray, and A. Wollock. 1984. Acute respiratory tract infections in children: a global public-health problem. New Engl. J. Med. 310:982-984. - PubMed
-
- Dachsner, F., U. Behre, and A. Dalhoff. 1981. Prospective clinical trial on the efficacy of amoxicillin administered twice or four times daily in children with respiratory tract infections. J. Int. Med. Res. 9:274-276. - PubMed
-
- Deeks, S., R. Palacio, R. Ruvinsky, D. Kertesz, M. Hortal, A. Rossi, J. Spika, J. Di Fabio, et al. 1999. Risk factors and course of illness among children with invasive penicillin-resistant Streptococcus pneumoniae. Pediatrics 103:409-413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

