A role of topoisomerase II in linking DNA replication to chromosome condensation
- PMID: 12604590
- PMCID: PMC2173373
- DOI: 10.1083/jcb.200209023
A role of topoisomerase II in linking DNA replication to chromosome condensation
Abstract
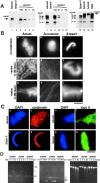
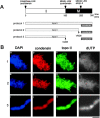
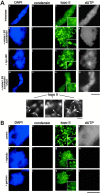
The condensin complex and topoisomerase II (topo II) have different biochemical activities in vitro, and both are required for mitotic chromosome condensation. We have used Xenopus egg extracts to investigate the functional interplay between condensin and topo II in chromosome condensation. When unreplicated chromatin is directly converted into chromosomes with single chromatids, the two proteins must function together, although they are independently targeted to chromosomes. In contrast, the requirement for topo II is temporarily separable from that of condensin when chromosome assembly is induced after DNA replication. This experimental setting allows us to find that, in the absence of condensin, topo II becomes enriched in an axial structure within uncondensed chromatin. Subsequent addition of condensin converts this structure into mitotic chromosomes in an ATP hydrolysis-dependent manner. Strikingly, preventing DNA replication by the addition of geminin or aphidicolin disturbs the formation of topo II-containing axes and alters the binding property of topo II with chromatin. Our results suggest that topo II plays an important role in an early stage of chromosome condensation, and that this function of topo II is tightly coupled with prior DNA replication.
Figures




Similar articles
-
Condensin-dependent rDNA decatenation introduces a temporal pattern to chromosome segregation.Curr Biol. 2008 Jul 22;18(14):1084-9. doi: 10.1016/j.cub.2008.06.058. Curr Biol. 2008. PMID: 18635352
-
Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II.J Virol. 2007 May;81(10):5166-80. doi: 10.1128/JVI.00120-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360754 Free PMC article.
-
Association of human DNA topoisomerase IIalpha with mitotic chromosomes in mammalian cells is independent of its catalytic activity.Exp Cell Res. 1999 Oct 10;252(1):50-62. doi: 10.1006/excr.1999.4616. Exp Cell Res. 1999. PMID: 10502399
-
Mitotic chromosome structure and condensation.Curr Opin Cell Biol. 2006 Dec;18(6):632-8. doi: 10.1016/j.ceb.2006.09.007. Epub 2006 Oct 12. Curr Opin Cell Biol. 2006. PMID: 17046228 Review.
-
Condensin and biological role of chromosome condensation.Prog Cell Cycle Res. 2003;5:361-7. Prog Cell Cycle Res. 2003. PMID: 14593730 Review.
Cited by
-
Maternal topoisomerase II alpha, not topoisomerase II beta, enables embryonic development of zebrafish top2a-/- mutants.BMC Dev Biol. 2011 Nov 23;11:71. doi: 10.1186/1471-213X-11-71. BMC Dev Biol. 2011. PMID: 22111588 Free PMC article.
-
The MukB-ParC interaction affects the intramolecular, not intermolecular, activities of topoisomerase IV.J Biol Chem. 2013 Mar 15;288(11):7653-7661. doi: 10.1074/jbc.M112.418087. Epub 2013 Jan 24. J Biol Chem. 2013. PMID: 23349462 Free PMC article.
-
Condensin II interacts with BLM helicase in S phase to maintain genome stability.Commun Biol. 2025 Mar 25;8(1):492. doi: 10.1038/s42003-025-07916-0. Commun Biol. 2025. PMID: 40133469 Free PMC article.
-
Condensin: crafting the chromosome landscape.Chromosoma. 2013 Jun;122(3):175-90. doi: 10.1007/s00412-013-0405-1. Epub 2013 Apr 2. Chromosoma. 2013. PMID: 23546018 Review.
-
DNA topoisomerase IIα controls replication origin cluster licensing and firing time in Xenopus egg extracts.Nucleic Acids Res. 2013 Aug;41(15):7313-31. doi: 10.1093/nar/gkt494. Epub 2013 Jun 11. Nucleic Acids Res. 2013. PMID: 23757188 Free PMC article.
References
-
- Adachi, Y., M. Luke, and U.K. Laemmli. 1991. Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell. 64:137–148. - PubMed
-
- Bazzett-Jones, D.P., K. Kimura, and T. Hirano. 2002. Efficient supercoiling of DNA by a single condensin complex as revealed by electron epectroscopic imaging. Mol. Cell. 9:1183–1190. - PubMed
-
- Bhat, M.A., A.V. Philp, D.M. Glover, and H.J. Bellen. 1996. Chromatid segregation at anaphase requires the barren product, a novel chromosome associated protein that interacts with topoisomerase II. Cell. 87:1103–1114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

