Src promotes destruction of c-Cbl: implications for oncogenic synergy between Src and growth factor receptors
- PMID: 12604776
- PMCID: PMC151359
- DOI: 10.1073/pnas.0437945100
Src promotes destruction of c-Cbl: implications for oncogenic synergy between Src and growth factor receptors
Abstract
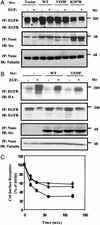
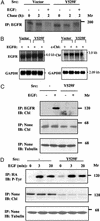
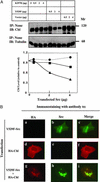
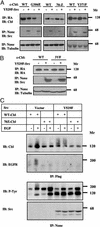
Cellular Src and epidermal growth factor receptor (EGFR) collaborate in the progression of certain human malignancies, and their cooverexpression characterizes relatively aggressive animal tumors. Our study addressed the mode of oncogenic cooperation and reports that overexpression of c-Src in model cellular systems results in the accumulation of EGFR at the cell surface. The underlying mechanism involves inhibition of the normal, c-Cbl-regulated process of ligand-induced receptor down-regulation. In response to activation of c-Src, c-Cbl proteins undergo tyrosine phosphorylation that promotes their ubiquitylation and proteasomal destruction. Consequently, ubiquitylation of EGFR by c-Cbl is restrained in Src-transformed cells, and receptor sorting to endocytosis is impaired. In conclusion, by promoting destruction of c-Cbl, c-Src enables EGFR to evade desensitization, which explains Src-EGFR collaboration in oncogenesis.
Figures

References
-
- Yarden Y, Sliwkowski M X. Nat Rev Mol Cell Biol. 2001;2:127–137. - PubMed
-
- Abram C L, Courtneidge S A. Exp Cell Res. 2000;254:1–13. - PubMed
-
- Biscardi J S, Tice D A, Parsons S J. Adv Cancer Res. 1999;76:61–119. - PubMed
-
- Irby R B, Mao W, Coppola D, Kang J, Loubeau J M, Trudeau W, Karl R, Fujita D J, Jove R, Yeatman T J. Nat Genet. 1999;21:187–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

