Induction of chemokines by low-dose intratracheal silica is reduced in TNFR I (p55) null mice
- PMID: 12604844
- PMCID: PMC2771223
- DOI: 10.1093/toxsci/kfg018
Induction of chemokines by low-dose intratracheal silica is reduced in TNFR I (p55) null mice
Abstract
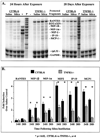
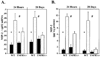
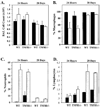
Previous studies suggest that tumor necrosis factor alpha (TNF-alpha) and the TNFRI (p55) and TNFRRII (p75) receptors mediate the pulmonary fibrotic response to silica. In order to further define the role of the TNFRI (p55) receptor in induction of profibrotic chemokines by low-dose silica/crystalline silica (50 micro g/50 micro l/mouse) or control diluent saline was instilled into the trachea of TNFRI gene ablated ((-/-)) and C57BL/6 (WT) control mice. Lung tissue was harvested and bronchoalveolar lavage (BAL) performed 24 h and 28 days following silica administration. Selected profibrotic chemokine mRNAs were quantified by ribonuclease protection assay, normalized to ribosomal protein L32 mRNA content and expressed relative to saline control treated lungs. Induction of MIP-1beta, MIP-1alpha, MIP-2, IP-10, and MCP-1 mRNAs was attenuated in the TNFRI(-/-) mice, in comparison to WT mice, particularly at 28 days after exposure. ELISA assays for MIP-1alpha and MIP-2 in homogenized lung tissue similarly demonstrated marked induction of both chemokines 24 h after silica treatment, which was persistent at 28 days in WT but not in TNFRI(-/-) mice. The percentage of BAL cells that was neutrophils was comparably increased in WT and RI(-/-) lungs at 24 h (49 +/- 12% vs. 46 +/- 10%) and 28 days (6.2 +/- 1.5% vs. 4.5 +/- 1%). The increase in total lavagable cells and BAL protein was also independent of strain. Histology revealed mild alveolitis without granuloma formation in both strains, slightly decreased in TNFRI(-/-). This study demonstrates an increase in pro-fibrotic chemokines in response to a single intratracheal exposure to crystalline silica that was sustained at 28 days after treatment in WT but not in TNFRI(-/-) mice. Silica dependent recruitment of neutrophils to the alveolar space and alveolar protein leak were, however, not altered by the absence of the TNF receptor.
Figures



Similar articles
-
Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice.J Leukoc Biol. 2004 Oct;76(4):886-95. doi: 10.1189/jlb.1203644. Epub 2004 Jul 7. J Leukoc Biol. 2004. PMID: 15240757
-
Ablation of tumor necrosis factor receptor type I (p55) alters oxygen-induced lung injury.Am J Physiol Lung Cell Mol Physiol. 2000 May;278(5):L1082-90. doi: 10.1152/ajplung.2000.278.5.L1082. Am J Physiol Lung Cell Mol Physiol. 2000. PMID: 10781441
-
MIP-1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF-alpha and LTB4.J Leukoc Biol. 2005 Jul;78(1):167-77. doi: 10.1189/jlb.0404237. Epub 2005 Apr 14. J Leukoc Biol. 2005. PMID: 15831559
-
Upregulation of the p75 but not the p55 TNF-alpha receptor mRNA after silica and bleomycin exposure and protection from lung injury in double receptor knockout mice.Am J Respir Cell Mol Biol. 1999 Apr;20(4):825-33. doi: 10.1165/ajrcmb.20.4.3193. Am J Respir Cell Mol Biol. 1999. PMID: 10101016
-
The role of chemokines and their receptors in the rejection of pig islet tissue xenografts.Xenotransplantation. 2003 Mar;10(2):164-77. doi: 10.1034/j.1399-3089.2003.01146.x. Xenotransplantation. 2003. PMID: 12588649
Cited by
-
Chronic Alcohol Exposure Enhances Lipopolysaccharide-Induced Lung Injury in Mice: Potential Role of Systemic Tumor Necrosis Factor-Alpha.Alcohol Clin Exp Res. 2015 Oct;39(10):1978-88. doi: 10.1111/acer.12855. Epub 2015 Sep 18. Alcohol Clin Exp Res. 2015. PMID: 26380957 Free PMC article.
-
Silica-directed mast cell activation is enhanced by scavenger receptors.Am J Respir Cell Mol Biol. 2007 Jan;36(1):43-52. doi: 10.1165/rcmb.2006-0197OC. Epub 2006 Aug 10. Am J Respir Cell Mol Biol. 2007. PMID: 16902192 Free PMC article.
-
Anti-TNFα therapy in inflammatory lung diseases.Pharmacol Ther. 2017 Dec;180:90-98. doi: 10.1016/j.pharmthera.2017.06.008. Epub 2017 Jun 19. Pharmacol Ther. 2017. PMID: 28642115 Free PMC article. Review.
-
Regulation of caveolin-1 expression, nitric oxide production and tissue injury by tumor necrosis factor-alpha following ozone inhalation.Toxicol Appl Pharmacol. 2008 Mar 15;227(3):380-9. doi: 10.1016/j.taap.2007.11.012. Epub 2007 Nov 22. Toxicol Appl Pharmacol. 2008. PMID: 18207479 Free PMC article.
-
Silica-induced chronic inflammation promotes lung carcinogenesis in the context of an immunosuppressive microenvironment.Neoplasia. 2013 Aug;15(8):913-24. doi: 10.1593/neo.13310. Neoplasia. 2013. PMID: 23908592 Free PMC article.
References
-
- Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Antioxidant treatment attenuates cytokine and chemokine levels in murine macrophages following silica exposure. Toxicol. Appl. Pharmacol. 1999a;158:211–220. - PubMed
-
- Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-α-induced oxidant stress. Am. J. Physiol. 1999b;276:L979–L988. - PubMed
-
- Claudio E, Segade F, Wrobel K, Ramos S, Lazo PS. Activation of murine macrophages by silica particles in vitro is a process independent of silica-induced cell death. Am. J. Respir. Cell Mol. Biol. 1995;13:547–554. - PubMed
-
- Cooper GS, Miller FW, Germolec DR. Occupational exposures and autoimmune diseases. Int. Immunopharmacol. 2002;2:303–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

