Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes
- PMID: 12606570
- PMCID: PMC150330
- DOI: 10.1093/emboj/cdg096
Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes
Abstract
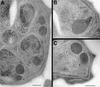
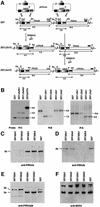
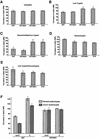
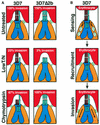
The members of the phylum Apicomplexa parasitize a wide range of eukaryotic host cells. Plasmodium falciparum, responsible for the most virulent form of malaria, invades human erythrocytes using several specific and high affinity ligand-receptor interactions that define invasion pathways. We find that members of the P. falciparum reticulocyte-binding homolog protein family, PfRh2a and PfRh2b, are expressed variantly in different lines. Targeted gene disruption shows that PfRh2b mediates a novel invasion pathway and that it functions independently of other related proteins. Phenotypic variation of the PfRh protein family allows P. falciparum to exploit different patterns of receptors on the erythrocyte surface and thereby respond to polymorphisms in erythrocyte receptors and to evade the host immune system.
Figures


References
-
- Adams J.H., Blair,P.L., Kaneko,O. and Peterson,D.S. (2001) An expanding ebl family of Plasmodium falciparum. Trends Parasitol., 17, 297–299. - PubMed
-
- Barnwell J.W. and Galinski,M.R. (1998) Invasion of vertebrate cells: erythrocytes. In Sherman,I.W. (ed.), Malaria: Parasite Biology, Pathogenesis and Protection. ASM Press, Washington, DC, pp. 193–120.
-
- Butcher G.A., Mitchell,G.H. and Cohen,S. (1973) Mechanism of host specificity in malarial infection. Nature, 244, 40–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

