FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts
- PMID: 12606579
- PMCID: PMC150349
- DOI: 10.1093/emboj/cdg116
FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts
Abstract
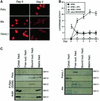
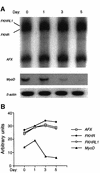
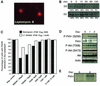
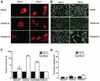
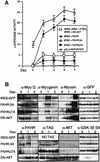
Activation of the transcription factor FKHR (Forkhead in human rhabdomyosarcoma, FOXO1a) in various established cell lines induces cell cycle arrest followed by apoptosis. These effects are inhibited through activation of the phosphatidylinositol 3-kinase/Akt pathway, resulting in FKHR phosphorylation and its export from the nucleus, thus blocking its pro-apoptotic activity. Here we report that FKHR regulates fusion of differentiating primary myoblasts. We demonstrate that FKHR is localized in the cytoplasm of proliferating myoblasts, yet translocates to the nucleus by a phosphorylation-independent pathway following serum starvation, a condition that induces myoblast differentiation. FKHR phosphorylation during terminal differentiation appears to downregulate its fusion activity, as a dominant-active non-phosphorylatable FKHR mutant dramatically augments the rate and extent of myotube fusion. However, this FKHR mutant exerts its effects only after other events initiated the differentiation pro cess. Conversely, enforced expression of a dominant-negative FKHR mutant blocks myotube formation whereas wild-type FKHR has no effect. We conclude that in addition to the role of FoxO proteins in regulating cell cycle progress and apoptosis, FKHR controls the rate of myotube fusion during myogenic differentiation.
Figures


References
-
- Alvarez B., Martinez,A.C., Burgering,B.M. and Carrera,A.C. (2001) Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature, 413, 744–747. - PubMed
-
- Arnold H.H. and Winter,B. (1998) Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev., 8, 539–544. - PubMed
-
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Smith,J.A. and Struhl,K. (2001) Current Protocols in Molecular Biology. John Wiley and Sons, Harvard.
-
- Barr F.G. (2001) Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene, 20, 5736–5746. - PubMed
-
- Bellacosa A., Chan,T.O., Ahmed,N.N., Datta,K., Malstrom,S., Stokoe,D., McCormick,F., Feng,J. and Tsichlis,P. (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene, 17, 313–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

