The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo
- PMID: 12606580
- PMCID: PMC150341
- DOI: 10.1093/emboj/cdg108
The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo
Abstract
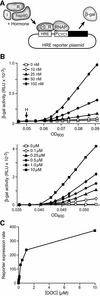
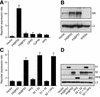
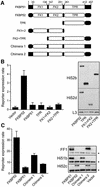
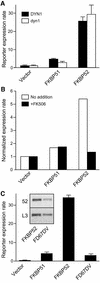
Hsp90 is required for the normal activity of steroid receptors, and in steroid receptor complexes it is typically bound to one of the immunophilin-related co-chaperones: the peptidylprolyl isomerases FKBP51, FKBP52 or CyP40, or the protein phosphatase PP5. The physiological roles of the immunophilins in regulating steroid receptor function have not been well defined, and so we examined in vivo the influences of immunophilins on hormone-dependent gene activation in the Saccharomyces cerevisiae model for glucocorticoid receptor (GR) function. FKBP52 selectively potentiates hormone-dependent reporter gene activation by as much as 20-fold at limiting hormone concentrations, and this potentiation is readily blocked by co-expression of the closely related FKBP51. The mechanism for potentiation is an increase in GR hormone-binding affinity that requires both the Hsp90-binding ability and the prolyl isomerase activity of FKBP52.
Figures



References
-
- Barent R.L., Nair,S.C., Carr,D.C., Ruan,Y., Rimerman,R.A., Fulton,J., Zhang,Y. and Smith,D.F. (1998) Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol., 12, 342–354. - PubMed
-
- Baughman G., Harrigan,M.T., Campbell,N.F., Nurrish,S.J. and Bourgeois,S. (1991) Genes newly identified as regulated by glucocorticoids in murine thymocytes. Mol. Endocrinol., 5, 637–644. - PubMed
-
- Bose S., Weikl,T., Bugl,H. and Buchner,J. (1996) Chaperone function of Hsp90-associated proteins. Science, 274, 1715–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

