HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo
- PMID: 12606581
- PMCID: PMC150348
- DOI: 10.1093/emboj/cdg115
HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo
Abstract
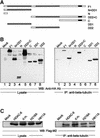
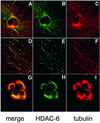
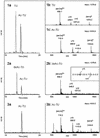
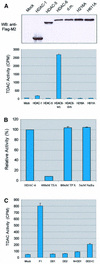
Microtubules are cylindrical cytoskeletal structures found in almost all eukaryotic cell types which are involved in a great variety of cellular processes. Reversible acetylation on the epsilon-amino group of alpha-tubulin Lys40 marks stabilized microtubule structures and may contribute to regulating microtubule dynamics. Yet, the enzymes catalysing this acetylation/deacetylation have remained unidentified until recently. Here we report that beta-tubulin interacts with histone deacetylase-6 (HDAC-6) in a yeast two-hybrid assay and in vitro. We find that HDAC-6 is a micro tubule-associated protein capable of deacetylating alpha-tubulin in vivo and in vitro. HDAC-6's microtubule binding and deacetylation functions both depend on the hdac domains. Overexpression of HDAC-6 in mammalian cells leads to tubulin hypoacetylation. In contrast, inhibition of HDAC-6 function by two independent mechanisms--pharmacological (HDAC inhibitors) or genetic (targeted inactivation of HDAC-6 in embryonic stem cells)--leads to hyperacetylation of tubulin and microtubules. Taken together, our data provide evidence that HDAC-6 might act as a dual deacetylase for tubulin and histones, and suggest the possibility that acetylated non-histone proteins might represent novel targets for pharmacological therapy by HDAC inhibitors.
Figures




References
-
- Adachi N., Kimura,A. and Horikoshi,M. (2002) A conserved motif common to the histone acetyltransferase Esa1 and the histone deacetylase Rpd3. J. Biol. Chem., 277, 35688–35695. - PubMed
-
- Brown C.E., Lechner,T., Howe,L. and Workman,J.L. (2000) The many HATs of transcription coactivators. Trends Biochem. Sci., 25, 15–19. - PubMed
-
- Cheung W.L., Briggs,S.D. and Allis,C.D. (2000) Acetylation and chromosomal functions. Curr. Opin. Cell Biol., 12, 326–333. - PubMed
-
- Fields S. and Song,O. (1989) A novel genetic system to detect protein–protein interactions. Nature, 340, 245–246. - PubMed
-
- Finnin M.S., Donigian,J.R., Cohen,A., Richon,V.M., Rifkind,R.A., Marks,P.A., Breslow,R. and Pavletich,N.P. (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature, 401, 188–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

